当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Absorption of Low-Energy UV Radiation by Human Telomere G-Quadruplexes Generates Long-Lived Guanine Radical Cations
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-07-24 , DOI: 10.1021/jacs.7b05931 Akos Banyasz 1 , Lara Martínez-Fernández 2 , Clémence Balty 1 , Marion Perron 1 , Thierry Douki 3 , Roberto Improta 1, 2 , Dimitra Markovitsi 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-07-24 , DOI: 10.1021/jacs.7b05931 Akos Banyasz 1 , Lara Martínez-Fernández 2 , Clémence Balty 1 , Marion Perron 1 , Thierry Douki 3 , Roberto Improta 1, 2 , Dimitra Markovitsi 1
Affiliation
|
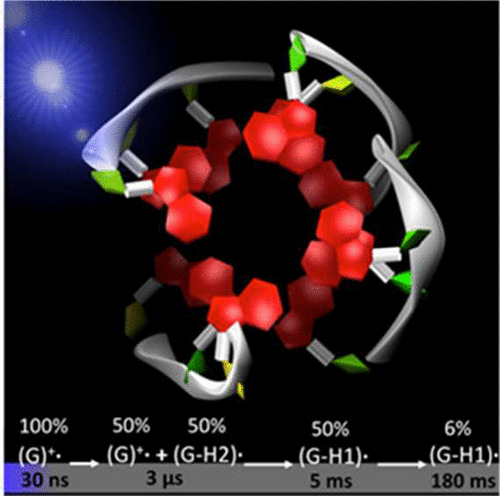
Telomeres, which are involved in cell division, carcinogenesis, and aging and constitute important therapeutic targets, are prone to oxidative damage. This propensity has been correlated with the presence of guanine-rich sequences, capable of forming four-stranded DNA structures (G-quadruplexes). Here, we present the first study on oxidative damage of human telomere G-quadruplexes without mediation of external molecules. Our investigation has been performed for G-quadruplexes formed by folding of GGG(TTAGGG)3 single strands in buffered solutions containing Na+ cations (TEL21/Na+). Associating nanosecond time-resolved spectroscopy and quantum mechanical calculations (TD-DFT), it focuses on the primary species, ejected electrons and guanine radicals, generated upon absorption of UV radiation directly by TEL21/Na+. We show that, at 266 nm, corresponding to an energy significantly lower than the guanine ionization potential, the one-photon ionization quantum yield is 4.5 × 10-3. This value is comparable to that of cyclobutane thymine dimers (the major UV-induced lesions) in genomic DNA; the quantum yield of these dimers in TEL21/Na+ is found to be (1.1 ± 0.1) × 10-3. The fate of guanine radicals, generated in equivalent concentration with that of ejected electrons, is followed over 5 orders of magnitude of time. Such a quantitative approach reveals that an important part of radical cation population survives up to a few milliseconds, whereas radical cations produced by chemical oxidants in various DNA systems are known to deprotonate, at most, within a few microseconds. Under the same experimental conditions, neither one-photon ionization nor long-lived radical cations are detected for the telomere repeat TTAGGG in single-stranded configuration, showing that secondary structure plays a key role in these processes. Finally, two types of deprotonated radicals are identified: on the one hand, (G-H2)• radicals, stable at early times, and on the other hand, (G-H1)• radicals, appearing within a few milliseconds and decaying with a time constant of ∼50 ms.
中文翻译:

人类端粒 G-四链体对低能紫外线辐射的吸收产生长寿命鸟嘌呤自由基阳离子
端粒参与细胞分裂、癌变和衰老并构成重要的治疗靶点,容易发生氧化损伤。这种倾向与富含鸟嘌呤的序列的存在相关,能够形成四链 DNA 结构(G-四链体)。在这里,我们首次在没有外部分子介导的情况下对人类端粒 G-四链体的氧化损伤进行了研究。我们的研究是针对 GGG(TTAGGG)3 单链在含有 Na+ 阳离子 (TEL21/Na+) 的缓冲溶液中折叠形成的 G-四链体进行的。将纳秒时间分辨光谱和量子力学计算 (TD-DFT) 相关联,它专注于主要物质、喷射电子和鸟嘌呤自由基,这些物质是由 TEL21/Na+ 直接吸收紫外线辐射产生的。我们表明,在 266 nm 处,对应于明显低于鸟嘌呤电离势的能量,单光子电离量子产率为 4.5 × 10-3。该值与基因组 DNA 中的环丁烷胸腺嘧啶二聚体(主要的紫外线诱导损伤)相当;发现这些二聚体在 TEL21/Na+ 中的量子产率为 (1.1 ± 0.1) × 10-3。鸟嘌呤自由基的命运,以与喷射电子的浓度相等的浓度产生,被跟踪超过 5 个数量级的时间。这种定量方法表明,自由基阳离子群体的重要组成部分最多可存活几毫秒,而已知由各种 DNA 系统中的化学氧化剂产生的自由基阳离子最多在几微秒内去质子化。在相同的实验条件下,对于单链配置的端粒重复 TTAGGG,既没有检测到单光子电离,也没有检测到长寿命自由基阳离子,表明二级结构在这些过程中起着关键作用。最后,确定了两种类型的去质子化自由基:一方面,(G-H2)• 自由基,早期稳定,另一方面,(G-H1)• 自由基,在几毫秒内出现并随着约 50 毫秒的时间常数。
更新日期:2017-07-24
中文翻译:

人类端粒 G-四链体对低能紫外线辐射的吸收产生长寿命鸟嘌呤自由基阳离子
端粒参与细胞分裂、癌变和衰老并构成重要的治疗靶点,容易发生氧化损伤。这种倾向与富含鸟嘌呤的序列的存在相关,能够形成四链 DNA 结构(G-四链体)。在这里,我们首次在没有外部分子介导的情况下对人类端粒 G-四链体的氧化损伤进行了研究。我们的研究是针对 GGG(TTAGGG)3 单链在含有 Na+ 阳离子 (TEL21/Na+) 的缓冲溶液中折叠形成的 G-四链体进行的。将纳秒时间分辨光谱和量子力学计算 (TD-DFT) 相关联,它专注于主要物质、喷射电子和鸟嘌呤自由基,这些物质是由 TEL21/Na+ 直接吸收紫外线辐射产生的。我们表明,在 266 nm 处,对应于明显低于鸟嘌呤电离势的能量,单光子电离量子产率为 4.5 × 10-3。该值与基因组 DNA 中的环丁烷胸腺嘧啶二聚体(主要的紫外线诱导损伤)相当;发现这些二聚体在 TEL21/Na+ 中的量子产率为 (1.1 ± 0.1) × 10-3。鸟嘌呤自由基的命运,以与喷射电子的浓度相等的浓度产生,被跟踪超过 5 个数量级的时间。这种定量方法表明,自由基阳离子群体的重要组成部分最多可存活几毫秒,而已知由各种 DNA 系统中的化学氧化剂产生的自由基阳离子最多在几微秒内去质子化。在相同的实验条件下,对于单链配置的端粒重复 TTAGGG,既没有检测到单光子电离,也没有检测到长寿命自由基阳离子,表明二级结构在这些过程中起着关键作用。最后,确定了两种类型的去质子化自由基:一方面,(G-H2)• 自由基,早期稳定,另一方面,(G-H1)• 自由基,在几毫秒内出现并随着约 50 毫秒的时间常数。


























 京公网安备 11010802027423号
京公网安备 11010802027423号