当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The n→π* Interaction
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-07-23 00:00:00 , DOI: 10.1021/acs.accounts.7b00121 Robert W. Newberry 1 , Ronald T. Raines 1, 2
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-07-23 00:00:00 , DOI: 10.1021/acs.accounts.7b00121 Robert W. Newberry 1 , Ronald T. Raines 1, 2
Affiliation
|
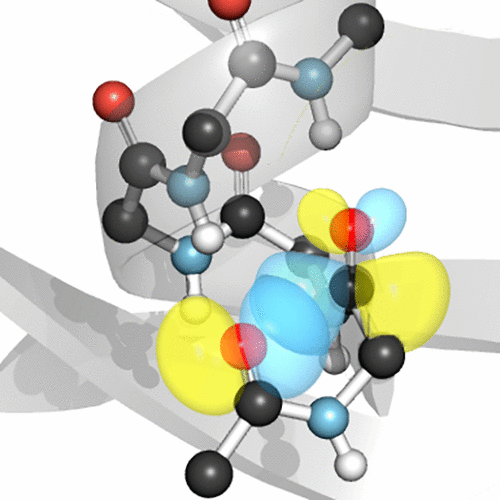
The carbonyl group holds a prominent position in chemistry and biology not only because it allows diverse transformations but also because it supports key intermolecular interactions, including hydrogen bonding. More recently, carbonyl groups have been found to interact with a variety of nucleophiles, including other carbonyl groups, in what we have termed an n→π* interaction. In an n→π* interaction, a nucleophile donates lone-pair (n) electron density into the empty π* orbital of a nearby carbonyl group. Mixing of these orbitals releases energy, resulting in an attractive interaction. Hints of such interactions were evident in small-molecule crystal structures as early as the 1970s, but not until 2001 was the role of such interactions articulated clearly.
中文翻译:

该ñ →π*互动
羰基在化学和生物学中占有重要地位,不仅因为它可以进行多种转化,还因为它支持关键的分子间相互作用,包括氢键。最近,我们发现羰基与包括其他羰基在内的各种亲核试剂发生相互作用,我们称之为n →π*相互作用。在n →π*相互作用中,亲核试剂将孤对(n)电子密度捐赠给空π *附近羰基的轨道。这些轨道的混合释放能量,从而产生有吸引力的相互作用。这种相互作用的提示早在1970年代就已经出现在小分子晶体结构中,但是直到2001年,这种相互作用的作用才被清楚地表达出来。
更新日期:2017-07-23
中文翻译:

该ñ →π*互动
羰基在化学和生物学中占有重要地位,不仅因为它可以进行多种转化,还因为它支持关键的分子间相互作用,包括氢键。最近,我们发现羰基与包括其他羰基在内的各种亲核试剂发生相互作用,我们称之为n →π*相互作用。在n →π*相互作用中,亲核试剂将孤对(n)电子密度捐赠给空π *附近羰基的轨道。这些轨道的混合释放能量,从而产生有吸引力的相互作用。这种相互作用的提示早在1970年代就已经出现在小分子晶体结构中,但是直到2001年,这种相互作用的作用才被清楚地表达出来。


























 京公网安备 11010802027423号
京公网安备 11010802027423号