当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Evaluation of Dimeric Derivatives of Diacylglycerol–Lactones as Protein Kinase C Ligands
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2017-07-21 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00299 Nami Ohashi 1 , Ryosuke Kobayashi 1 , Wataru Nomura 1 , Takuya Kobayakawa 1 , Agnes Czikora 2 , Brienna K. Herold 2 , Nancy E. Lewin 2 , Peter M. Blumberg 2 , Hirokazu Tamamura 1
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2017-07-21 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00299 Nami Ohashi 1 , Ryosuke Kobayashi 1 , Wataru Nomura 1 , Takuya Kobayakawa 1 , Agnes Czikora 2 , Brienna K. Herold 2 , Nancy E. Lewin 2 , Peter M. Blumberg 2 , Hirokazu Tamamura 1
Affiliation
|
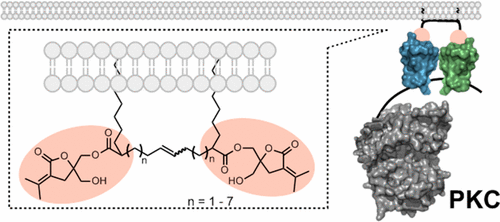
Protein kinase C (PKC) mediates a central cellular signal transduction pathway involved in disorders such as cancer and Alzheimer’s disease. PKC is regulated by binding of the second messenger sn-1,2-diacylglycerol (DAG) to its tandem C1 domains, designated C1a and C1b, leading both to PKC activation and to its translocation to the plasma membrane and to internal organelles. Depending on the isoform, there may be differences in the ligand selectivity of the C1a and C1b domains, and there is different spacing between the C1 domains of the conventional and novel PKCs. Bivalent ligands have the potential to exploit these differences between isoforms, yielding isoform selectivity. In the present study, we describe the synthesis of a series of dimeric derivatives of conformationally constrained diacylglycerol (DAG) analogs (DAG–lactones). We characterize the derivatives in vitro for their binding affinities, both to a single C1 domain (the C1b domain of PKCδ) as well as to the conventional PKCα isoform and the novel PKCδ isoform, and we measure their abilities to cause translocation of PKCδ and PKCε in intact cells. The dimeric compound with the 10-carbon linker was modestly more effective for the isolated PKCδ C1b domain than was the monomeric compound. For the intact PKCα and PKCδ, the shortest DAG–lactone dimer had similar affinity to the monomer and affinity decreased progressively up to the 16-carbon linker. The dimeric derivatives did not cause the Golgi accumulation of PKCδ. The present results provide important insights into the development of new chemical tools for biological studies on PKC.
中文翻译:

二酰基甘油-内酯二聚体衍生物作为蛋白激酶C配体的合成与评价
蛋白激酶C(PKC)介导涉及诸如癌症和阿尔茨海默氏病等疾病的中央细胞信号转导途径。PKC受第二信使sn绑定的约束-1,2-二酰基甘油(DAG)到达其串联C1域,称为C1a和C1b,从而导致PKC活化并转移至质膜和内部细胞器。取决于同工型,C1a和C1b结构域的配体选择性可能有所不同,并且传统PKC和新型PKC的C1结构域之间的间距也不同。二价配体具有利用同工型之间这些差异的潜力,从而产生同工型选择性。在本研究中,我们描述了一系列构象受限的二酰基甘油(DAG)类似物(DAG-内酯)的二聚体衍生物的合成。我们在体外对衍生物的结合亲和力进行了表征,它们既与单个C1域(PKCδ的C1b域)结合,也与常规PKCα同种型和新型PKCδ同种型结合,我们测量它们在完整细胞中引起PKCδ和PKCε易位的能力。具有10个碳原子的连接基的二聚化合物对分离的PKCδC1b结构域的影响要比单体化合物适度。对于完整的PKCα和PKCδ,最短的DAG-内酯二聚体与单体的亲和力相似,并且直到16个碳的连接基,亲和力逐渐降低。二聚体衍生物不引起PKCδ的高尔基积累。目前的结果提供了重要的见解,以开发用于PKC的生物学研究的新化学工具。最短的DAG-内酯二聚体具有与单体相似的亲和力,并且亲和力逐渐降低,直至达到16碳接头。二聚体衍生物不引起PKCδ的高尔基积累。目前的结果提供了重要的见解,以开发用于PKC的生物学研究的新化学工具。最短的DAG-内酯二聚体具有与单体相似的亲和力,并且亲和力逐渐降低,直至达到16碳接头。二聚体衍生物不引起PKCδ的高尔基积累。目前的结果提供了重要的见解,以开发用于PKC的生物学研究的新化学工具。
更新日期:2017-07-22
中文翻译:

二酰基甘油-内酯二聚体衍生物作为蛋白激酶C配体的合成与评价
蛋白激酶C(PKC)介导涉及诸如癌症和阿尔茨海默氏病等疾病的中央细胞信号转导途径。PKC受第二信使sn绑定的约束-1,2-二酰基甘油(DAG)到达其串联C1域,称为C1a和C1b,从而导致PKC活化并转移至质膜和内部细胞器。取决于同工型,C1a和C1b结构域的配体选择性可能有所不同,并且传统PKC和新型PKC的C1结构域之间的间距也不同。二价配体具有利用同工型之间这些差异的潜力,从而产生同工型选择性。在本研究中,我们描述了一系列构象受限的二酰基甘油(DAG)类似物(DAG-内酯)的二聚体衍生物的合成。我们在体外对衍生物的结合亲和力进行了表征,它们既与单个C1域(PKCδ的C1b域)结合,也与常规PKCα同种型和新型PKCδ同种型结合,我们测量它们在完整细胞中引起PKCδ和PKCε易位的能力。具有10个碳原子的连接基的二聚化合物对分离的PKCδC1b结构域的影响要比单体化合物适度。对于完整的PKCα和PKCδ,最短的DAG-内酯二聚体与单体的亲和力相似,并且直到16个碳的连接基,亲和力逐渐降低。二聚体衍生物不引起PKCδ的高尔基积累。目前的结果提供了重要的见解,以开发用于PKC的生物学研究的新化学工具。最短的DAG-内酯二聚体具有与单体相似的亲和力,并且亲和力逐渐降低,直至达到16碳接头。二聚体衍生物不引起PKCδ的高尔基积累。目前的结果提供了重要的见解,以开发用于PKC的生物学研究的新化学工具。最短的DAG-内酯二聚体具有与单体相似的亲和力,并且亲和力逐渐降低,直至达到16碳接头。二聚体衍生物不引起PKCδ的高尔基积累。目前的结果提供了重要的见解,以开发用于PKC的生物学研究的新化学工具。


























 京公网安备 11010802027423号
京公网安备 11010802027423号