当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
5-Formyl- and 5-Carboxydeoxycytidines Do Not Cause Accumulation of Harmful Repair Intermediates in Stem Cells
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-07-21 00:00:00 , DOI: 10.1021/jacs.7b04131 René Rahimoff 1 , Olesea Kosmatchev 1 , Angie Kirchner 1 , Toni Pfaffeneder 1 , Fabio Spada 1 , Victor Brantl 1 , Markus Müller 1 , Thomas Carell 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-07-21 00:00:00 , DOI: 10.1021/jacs.7b04131 René Rahimoff 1 , Olesea Kosmatchev 1 , Angie Kirchner 1 , Toni Pfaffeneder 1 , Fabio Spada 1 , Victor Brantl 1 , Markus Müller 1 , Thomas Carell 1
Affiliation
|
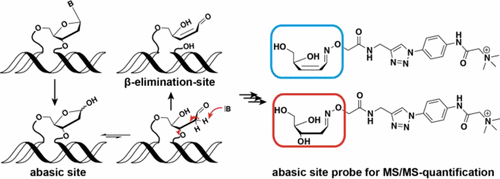
5-Formyl-dC (fdC) and 5-carboxy-dC (cadC) are newly discovered bases in the mammalian genome that are supposed to be substrates for base excision repair (BER) in the framework of active demethylation. The bases are recognized by the monofunctional thymine DNA glycosylase (Tdg), which cleaves the glycosidic bond of the bases to give potentially harmful abasic sites (AP-sites). Because of the turnover of fdC and cadC during cell state transitions, it is an open question to what extent such harmful AP-sites may accumulate during these processes. Here, we report the development of a new reagent that in combination with mass spectrometry (MS) allows us to quantify the levels of AP-sites. This combination also allowed the quantification of β-elimination (βE) products, which are repair intermediates of bifunctional DNA glycosylases. In combination with feeding of isotopically labeled nucleosides, we were able to trace the intermediates back to their original nucleobases. We show that, while the steady-state levels of fdC and cadC are substantially increased in Tdg-deficient cells, those of both AP- and βE-sites are unaltered. The levels of the detected BER intermediates are 1 and 2 orders of magnitude lower than those of cadC and fdC, respectively. Thus, neither the presence of fdC nor that of cadC in stem cells leads to the accumulation of harmful AP- and βE-site intermediates.
中文翻译:

5-甲酰基和5-羧基脱氧胞苷不会导致干细胞中有害修复中间体的积累
5-甲酰基-dC(fdC)和5-羧基-dC(cadC)是哺乳动物基因组中新发现的碱基,在活跃的脱甲基作用下,被认为是碱基切除修复(BER)的底物。碱基被单功能胸腺嘧啶DNA糖基化酶(Tdg)识别,该酶可裂解碱基的糖苷键以产生潜在有害的无碱基位点(AP位点)。由于细胞状态转换过程中fdC和cadC的更新,这是一个尚待解决的问题,这些有害的AP站点在这些过程中可能累积到什么程度。在这里,我们报告了一种新试剂的开发,该试剂与质谱(MS)结合使我们能够量化AP位点的水平。这种组合还可以定量分析β-消除(βE)产物,该产物是双功能DNA糖基化酶的修复中间体。结合饲喂同位素标记的核苷,我们能够将中间体追溯到其原始核苷碱基。我们显示,虽然在Tdg缺陷型细胞中fdC和cadC的稳态水平显着提高,但AP和βE站点的稳态水平均未改变。所检测到的BER中间产物的水平分别比cadC和fdC低1个数量级和2个数量级。因此,干细胞中fdC或cadC的存在均不会导致有害的AP和βE位点中间体的积累。所检测到的BER中间产物的水平分别比cadC和fdC低1个数量级和2个数量级。因此,干细胞中fdC或cadC的存在均不会导致有害的AP和βE位点中间体的积累。所检测到的BER中间产物的水平分别比cadC和fdC低1个数量级和2个数量级。因此,干细胞中fdC或cadC的存在均不会导致有害的AP和βE位点中间体的积累。
更新日期:2017-07-22
中文翻译:

5-甲酰基和5-羧基脱氧胞苷不会导致干细胞中有害修复中间体的积累
5-甲酰基-dC(fdC)和5-羧基-dC(cadC)是哺乳动物基因组中新发现的碱基,在活跃的脱甲基作用下,被认为是碱基切除修复(BER)的底物。碱基被单功能胸腺嘧啶DNA糖基化酶(Tdg)识别,该酶可裂解碱基的糖苷键以产生潜在有害的无碱基位点(AP位点)。由于细胞状态转换过程中fdC和cadC的更新,这是一个尚待解决的问题,这些有害的AP站点在这些过程中可能累积到什么程度。在这里,我们报告了一种新试剂的开发,该试剂与质谱(MS)结合使我们能够量化AP位点的水平。这种组合还可以定量分析β-消除(βE)产物,该产物是双功能DNA糖基化酶的修复中间体。结合饲喂同位素标记的核苷,我们能够将中间体追溯到其原始核苷碱基。我们显示,虽然在Tdg缺陷型细胞中fdC和cadC的稳态水平显着提高,但AP和βE站点的稳态水平均未改变。所检测到的BER中间产物的水平分别比cadC和fdC低1个数量级和2个数量级。因此,干细胞中fdC或cadC的存在均不会导致有害的AP和βE位点中间体的积累。所检测到的BER中间产物的水平分别比cadC和fdC低1个数量级和2个数量级。因此,干细胞中fdC或cadC的存在均不会导致有害的AP和βE位点中间体的积累。所检测到的BER中间产物的水平分别比cadC和fdC低1个数量级和2个数量级。因此,干细胞中fdC或cadC的存在均不会导致有害的AP和βE位点中间体的积累。



























 京公网安备 11010802027423号
京公网安备 11010802027423号