当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Conformation-Based Design and Synthesis of Apratoxin A Mimetics Modified at the α,β-Unsaturated Thiazoline Moiety
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-07-20 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00833 Yuichi Onda 1, 2 , Yuichi Masuda 1 , Masahito Yoshida 1 , Takayuki Doi 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-07-20 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00833 Yuichi Onda 1, 2 , Yuichi Masuda 1 , Masahito Yoshida 1 , Takayuki Doi 1
Affiliation
|
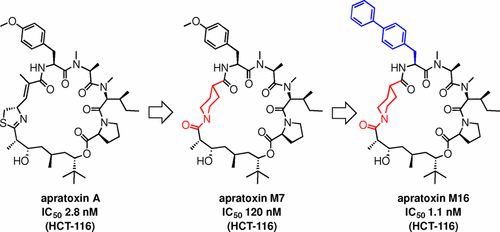
We have demonstrated design, synthesis, and biological evaluation of apratoxin A mimetics. In the first generation, the moCys moiety was replaced with seven simple amino acids as their 3D structures can be similar to that of apratoxin A. Apratoxins M1–M7 were synthesized using solid-phase peptide synthesis and solution-phase macrolactamization. Apratoxin M7, which contains a piperidinecarboxylic acid moiety, exhibited potent cytotoxicity against HCT-116 cells. In the second generation, substitution of each amino acid residue in the tripeptide Tyr(Me)–MeAla–MeIle moiety in apratoxin M7 led to the development of the highly potent apratoxin M16 possessing biphenylalanine (Bph) instead of Tyr(Me), which exhibited an IC50 value of 1.1 nM against HCT-116 cells. Moreover, compared to apratoxin A, apratoxin M16 exhibited a similarly high level of growth inhibitory activity against various cancer cell lines. The results indicate that apratoxin M16 could be a potential candidate as an anticancer agent.
中文翻译:

基于构象的设计和合成α,β-不饱和噻唑啉部分的Apratoxin A模拟物
我们已经证明了拟毒素A模拟物的设计,合成和生物学评估。在第一代中,moCys部分被七个简单的氨基酸取代,因为它们的3D结构可以与Apratoxin A相似。ApratoxinsM1-M7是通过固相肽合成和溶液相大内酰胺化作用合成的。含有哌啶羧酸部分的Apratoxin M7对HCT-116细胞表现出强力的细胞毒性。在第二代,Apratoxin M7的三肽Tyr(Me)–MeAla–MeIle部分中的每个氨基酸残基的取代导致开发了具有联苯丙氨酸(Bph)而不是Tyr(Me)的高效Apratoxin M16,其表现出IC 50对HCT-116细胞的检测值为1.1 nM。此外,与Apratoxin A相比,Apratoxin M16对各种癌细胞系表现出相似的高水平的生长抑制活性。结果表明,Apratoxin M16作为抗癌剂可能是潜在的候选药物。
更新日期:2017-07-21
中文翻译:

基于构象的设计和合成α,β-不饱和噻唑啉部分的Apratoxin A模拟物
我们已经证明了拟毒素A模拟物的设计,合成和生物学评估。在第一代中,moCys部分被七个简单的氨基酸取代,因为它们的3D结构可以与Apratoxin A相似。ApratoxinsM1-M7是通过固相肽合成和溶液相大内酰胺化作用合成的。含有哌啶羧酸部分的Apratoxin M7对HCT-116细胞表现出强力的细胞毒性。在第二代,Apratoxin M7的三肽Tyr(Me)–MeAla–MeIle部分中的每个氨基酸残基的取代导致开发了具有联苯丙氨酸(Bph)而不是Tyr(Me)的高效Apratoxin M16,其表现出IC 50对HCT-116细胞的检测值为1.1 nM。此外,与Apratoxin A相比,Apratoxin M16对各种癌细胞系表现出相似的高水平的生长抑制活性。结果表明,Apratoxin M16作为抗癌剂可能是潜在的候选药物。

























 京公网安备 11010802027423号
京公网安备 11010802027423号