当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pancake Bond Orders of a Series of π-Stacked Triangulene Radicals
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2017-07-19 06:15:36 , DOI: 10.1002/anie.201704941 Zhongyu Mou 1 , Miklos Kertesz 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2017-07-19 06:15:36 , DOI: 10.1002/anie.201704941 Zhongyu Mou 1 , Miklos Kertesz 1
Affiliation
|
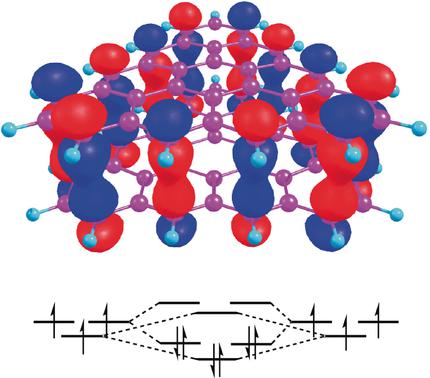
Conjugated radicals are capable of forming π-stacking “pancake-bonded” dimers. Members of the family of triangulene hydrocarbons, non-Kekulé neutral multiradicals, can utilize more than one singly occupied molecular orbital (SOMO) to form multiple pancake-bonded dimers with formal bond orders of up to five. The resulting dimer binding energies can be quite high and the intermolecular contacts rather small compared to the respective van der Waals values. The preferred configurations are driven by the large stabilization energy of overlapping SOMOs.
中文翻译:

一系列π堆积的Triangulene自由基的煎饼键阶
共轭自由基能够形成π堆积的“薄煎饼结合”二聚体。Triangulene碳氢化合物家族的成员,非Kekulé中性多自由基,可以利用一个以上的单占据分子轨道(SOMO)形成多个薄饼键合的二聚体,其正式键合阶数最多为5。与相应的范德华力值相比,所得的二聚体结合能可能很高,而分子间的接触相当小。首选的配置是由重叠SOMO的高稳定能量驱动的。
更新日期:2017-07-20
中文翻译:

一系列π堆积的Triangulene自由基的煎饼键阶
共轭自由基能够形成π堆积的“薄煎饼结合”二聚体。Triangulene碳氢化合物家族的成员,非Kekulé中性多自由基,可以利用一个以上的单占据分子轨道(SOMO)形成多个薄饼键合的二聚体,其正式键合阶数最多为5。与相应的范德华力值相比,所得的二聚体结合能可能很高,而分子间的接触相当小。首选的配置是由重叠SOMO的高稳定能量驱动的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号