当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemical Approaches to Investigate Labile Peptide and Protein Phosphorylation
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-07-19 00:00:00 , DOI: 10.1021/acs.accounts.7b00170 Anett Hauser 1, 2 , Martin Penkert 1, 2 , Christian P. R. Hackenberger 1, 2
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-07-19 00:00:00 , DOI: 10.1021/acs.accounts.7b00170 Anett Hauser 1, 2 , Martin Penkert 1, 2 , Christian P. R. Hackenberger 1, 2
Affiliation
|
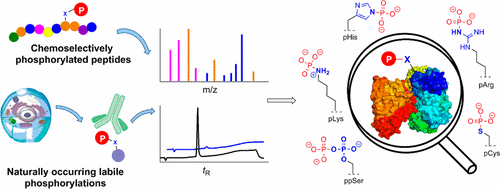
Protein phosphorylation is by far the most abundant and most studied post-translational modification (PTM). For a long time, phosphate monoesters of serine (pSer), threonine (pThr), and tyrosine (pTyr) have been considered as the only relevant forms of phosphorylation in organisms. Recently, several research groups have dedicated their efforts to the investigation of other, less characterized phosphoamino acids as naturally occurring PTMs. Such apparent peculiar phosphorylations include the phosphoramidates of histidine (pHis), arginine (pArg), and lysine (pLys), the phosphorothioate of cysteine (pCys), and the anhydrides of pyrophosphorylated serine (ppSer) and threonine (ppThr). Almost all of these phosphorylated amino acids show higher lability under physiological conditions than those of phosphate monoesters. Furthermore, they are prone to hydrolysis under acidic and sometimes basic conditions as well as at elevated temperatures, which renders their synthetic accessibility and proteomic analysis particularly challenging.
中文翻译:

调查不稳定肽和蛋白质磷酸化的化学方法
迄今为止,蛋白质磷酸化是最丰富和研究最多的翻译后修饰(PTM)。长期以来,丝氨酸(pSer),苏氨酸(pThr)和酪氨酸(pTyr)的磷酸单酯被认为是生物体中磷酸化的唯一相关形式。最近,几个研究小组已致力于研究其他较少表征的作为天然存在的PTM的磷酸氨基酸。这种明显的特殊磷酸化包括组氨酸(pHis),精氨酸(pArg)和赖氨酸(pLys)的氨基磷酸酯,半胱氨酸的硫代磷酸酯(pCys)和焦磷酸丝氨酸(ppSer)和苏氨酸的酸酐(ppThr)。几乎所有这些磷酸化氨基酸在生理条件下均比磷酸单酯具有更高的不稳定性。此外,
更新日期:2017-07-19
中文翻译:

调查不稳定肽和蛋白质磷酸化的化学方法
迄今为止,蛋白质磷酸化是最丰富和研究最多的翻译后修饰(PTM)。长期以来,丝氨酸(pSer),苏氨酸(pThr)和酪氨酸(pTyr)的磷酸单酯被认为是生物体中磷酸化的唯一相关形式。最近,几个研究小组已致力于研究其他较少表征的作为天然存在的PTM的磷酸氨基酸。这种明显的特殊磷酸化包括组氨酸(pHis),精氨酸(pArg)和赖氨酸(pLys)的氨基磷酸酯,半胱氨酸的硫代磷酸酯(pCys)和焦磷酸丝氨酸(ppSer)和苏氨酸的酸酐(ppThr)。几乎所有这些磷酸化氨基酸在生理条件下均比磷酸单酯具有更高的不稳定性。此外,



























 京公网安备 11010802027423号
京公网安备 11010802027423号