当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Developing an Asymmetric Transfer Hydrogenation Process for (S)-5-Fluoro-3-methylisobenzofuran-1(3H)-one, a Key Intermediate to Lorlatinib
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-07-19 00:00:00 , DOI: 10.1021/acs.oprd.7b00187 Shengquan Duan 1 , Bryan Li 1 , Robert W. Dugger 1 , Brian Conway 1 , Rajesh Kumar 1 , Carlos Martinez 1 , Teresa Makowski 1 , Robert Pearson 1 , Mark Olivier 1 , Roberto Colon-Cruz 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-07-19 00:00:00 , DOI: 10.1021/acs.oprd.7b00187 Shengquan Duan 1 , Bryan Li 1 , Robert W. Dugger 1 , Brian Conway 1 , Rajesh Kumar 1 , Carlos Martinez 1 , Teresa Makowski 1 , Robert Pearson 1 , Mark Olivier 1 , Roberto Colon-Cruz 1
Affiliation
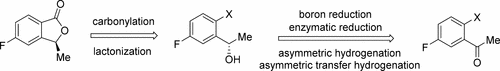
|
Synthesis of (S)-5-fluoro-3-methylisobenzofuran-1(3H)-one (6), a key intermediate to lorlatinib, is described. A few synthetic methodologies, that is, boron reduction, enzymatic reduction, asymmetric hydrogenation, and asymmetric transfer hydrogenation, were evaluated for the chiral reduction of the substituted acetophenone intermediate (8). A manufacturing process, on the basis of the asymmetric transfer hydrogenation, was developed. This process was successfully scaled up to prepare 400 kg of 6.
中文翻译:

开发不对称转移加氢工艺的(S)-5-氟-3-甲基异苯并呋喃-1(3 H)-一,洛拉替尼的关键中间体
描述了(S)-5-氟-3-甲基异苯并呋喃-1(3 H)-一(6)的合成,这是洛来替尼的关键中间体。对于取代的苯乙酮中间体的手性还原,评估了几种合成方法,即硼还原,酶还原,不对称氢化和不对称转移氢化(8)。在不对称转移氢化的基础上,开发了一种制造方法。此过程已成功扩大规模,以制备400公斤6。
更新日期:2017-07-20
中文翻译:

开发不对称转移加氢工艺的(S)-5-氟-3-甲基异苯并呋喃-1(3 H)-一,洛拉替尼的关键中间体
描述了(S)-5-氟-3-甲基异苯并呋喃-1(3 H)-一(6)的合成,这是洛来替尼的关键中间体。对于取代的苯乙酮中间体的手性还原,评估了几种合成方法,即硼还原,酶还原,不对称氢化和不对称转移氢化(8)。在不对称转移氢化的基础上,开发了一种制造方法。此过程已成功扩大规模,以制备400公斤6。



























 京公网安备 11010802027423号
京公网安备 11010802027423号