当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Challenges and Hallmarks of Establishing Alkylacetylphosphonates as Probes of Bacterial 1-Deoxy-d-xylulose 5-Phosphate Synthase
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2017-06-21 00:00:00 , DOI: 10.1021/acsinfecdis.6b00168 Sara Sanders 1 , Ryan J. Vierling 1 , David Bartee 1 , Alicia A. DeColli 1 , Mackenzie J. Harrison 2 , Joseph L. Aklinski 2 , Andrew T. Koppisch 2 , Caren L. Freel Meyers 1
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2017-06-21 00:00:00 , DOI: 10.1021/acsinfecdis.6b00168 Sara Sanders 1 , Ryan J. Vierling 1 , David Bartee 1 , Alicia A. DeColli 1 , Mackenzie J. Harrison 2 , Joseph L. Aklinski 2 , Andrew T. Koppisch 2 , Caren L. Freel Meyers 1
Affiliation
|
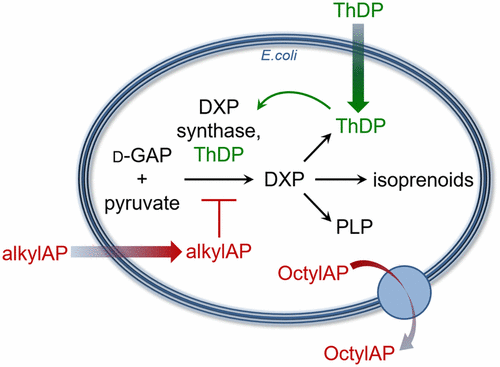
1-Deoxy-d-xylulose 5-phosphate (DXP) synthase catalyzes the thiamin diphosphate (ThDP)-dependent formation of DXP from pyruvate and d-glyceraldehyde 3-phosphate. DXP is at a metabolic branch point in bacteria, feeding into the methylerythritol phosphate pathway to indispensable isoprenoids and acting as a precursor for biosynthesis of essential cofactors in central metabolism, pyridoxal phosphate and ThDP, the latter of which is also required for DXP synthase catalysis. DXP synthase follows a unique random sequential mechanism and possesses an unusually large active site. These features have guided the design of sterically demanding alkylacetylphosphonates (alkylAPs) toward the development of selective DXP synthase inhibitors. alkylAPs studied here display selective, low μM inhibitory activity against DXP synthase. They are weak inhibitors of bacterial growth in standard nutrient rich conditions. However, bacteria are significantly sensitized to most alkylAPs in defined minimal growth medium, with minimal inhibitory concentrations (MICs) ranging from low μM to low mM and influenced by alkyl-chain length. The longest analog (C8) displays the weakest antimicrobial activity and is a substrate for efflux via AcrAB-TolC. The dependence of inhibitor potency on growth environment emphasizes the need for antimicrobial screening conditions that are relevant to the in vivo microbial microenvironment during infection. DXP synthase expression and thiamin supplementation studies offer support for DXP synthase as an intracellular target for some alkylAPs and reveal both the challenges and intriguing aspects of these approaches to study target engagement.
中文翻译:

建立烷基乙酰基膦酸酯作为细菌1-脱氧-d-木酮糖5-磷酸合酶探针的挑战和标志
1-脱氧d -xylulose -5-磷酸(DXP)合酶催化二磷酸硫胺(THDP)从丙酮酸和DXP的依赖性形成d-3-磷酸甘油醛。DXP处于细菌的代谢分支点,进入甲基赤藓糖醇磷酸途径到达必不可少的类异戊二烯,并充当中央代谢,吡ido醛磷酸盐和ThDP的重要辅因子生物合成的前体,后者对于DXP合酶催化也是必需的。DXP合酶遵循独特的随机顺序机制,并具有异常大的活性位点。这些特征已指导了对空间需求量较高的烷基乙酰基膦酸酯(烷基AP)的设计,以开发选择性DXP合酶抑制剂。本文研究的烷基AP对DXP合酶具有选择性,低μM的抑制活性。它们是标准养分丰富条件下细菌生长的弱抑制剂。然而,在限定的最小生长培养基中,细菌对大多数烷基AP显着敏感,其最小抑制浓度(MIC)在低μM至低mM范围内,并受烷基链长度的影响。最长的模拟量(C8)显示最弱的抗菌活性,并且是通过AcrAB-TolC流出的底物。抑制剂效能对生长环境的依赖性强调了对感染期间与体内微生物微环境有关的抗菌筛选条件的需求。DXP合酶表达和硫胺素补充研究为DXP合酶作为某些烷基AP的细胞内靶标提供了支持,并揭示了这些研究靶标参与的方法所面临的挑战和令人感兴趣的方面。
更新日期:2017-06-21
中文翻译:

建立烷基乙酰基膦酸酯作为细菌1-脱氧-d-木酮糖5-磷酸合酶探针的挑战和标志
1-脱氧d -xylulose -5-磷酸(DXP)合酶催化二磷酸硫胺(THDP)从丙酮酸和DXP的依赖性形成d-3-磷酸甘油醛。DXP处于细菌的代谢分支点,进入甲基赤藓糖醇磷酸途径到达必不可少的类异戊二烯,并充当中央代谢,吡ido醛磷酸盐和ThDP的重要辅因子生物合成的前体,后者对于DXP合酶催化也是必需的。DXP合酶遵循独特的随机顺序机制,并具有异常大的活性位点。这些特征已指导了对空间需求量较高的烷基乙酰基膦酸酯(烷基AP)的设计,以开发选择性DXP合酶抑制剂。本文研究的烷基AP对DXP合酶具有选择性,低μM的抑制活性。它们是标准养分丰富条件下细菌生长的弱抑制剂。然而,在限定的最小生长培养基中,细菌对大多数烷基AP显着敏感,其最小抑制浓度(MIC)在低μM至低mM范围内,并受烷基链长度的影响。最长的模拟量(C8)显示最弱的抗菌活性,并且是通过AcrAB-TolC流出的底物。抑制剂效能对生长环境的依赖性强调了对感染期间与体内微生物微环境有关的抗菌筛选条件的需求。DXP合酶表达和硫胺素补充研究为DXP合酶作为某些烷基AP的细胞内靶标提供了支持,并揭示了这些研究靶标参与的方法所面临的挑战和令人感兴趣的方面。


























 京公网安备 11010802027423号
京公网安备 11010802027423号