当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Utilization of an Active Site Mutant Receptor for the Identification of Potent and Selective Atypical 5-HT2C Receptor Agonists
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-07-07 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00385 Joseph Carpenter 1 , Ying Wang 1 , Gang Wu 1 , Jianxin Feng 1 , Xiang-Yang Ye 1 , Christian L. Morales 1 , Matthias Broekema 1 , Karen A. Rossi 1 , Keith J. Miller 1 , Brian J. Murphy 1 , Ginger Wu 1 , Sarah E. Malmstrom 1 , Anthony V. Azzara 1 , Philip M. Sher 1 , John M. Fevig 1 , Andrew Alt 1 , Robert L. Bertekap 1 , Mary Jane Cullen 1 , Timothy M. Harper 1 , Kimberly Foster 1 , Emily Luk 1 , Qian Xiang 1 , Mary F. Grubb 1 , Jeffrey A. Robl 1 , Dean A. Wacker 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-07-07 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00385 Joseph Carpenter 1 , Ying Wang 1 , Gang Wu 1 , Jianxin Feng 1 , Xiang-Yang Ye 1 , Christian L. Morales 1 , Matthias Broekema 1 , Karen A. Rossi 1 , Keith J. Miller 1 , Brian J. Murphy 1 , Ginger Wu 1 , Sarah E. Malmstrom 1 , Anthony V. Azzara 1 , Philip M. Sher 1 , John M. Fevig 1 , Andrew Alt 1 , Robert L. Bertekap 1 , Mary Jane Cullen 1 , Timothy M. Harper 1 , Kimberly Foster 1 , Emily Luk 1 , Qian Xiang 1 , Mary F. Grubb 1 , Jeffrey A. Robl 1 , Dean A. Wacker 1
Affiliation
|
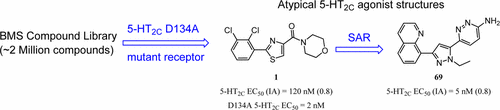
Agonism of the 5-HT2C receptor represents one of the most well-studied and clinically proven mechanisms for pharmacological weight reduction. Selectivity over the closely related 5-HT2A and 5-HT2B receptors is critical as their activation has been shown to lead to undesirable side effects and major safety concerns. In this communication, we report the development of a new screening paradigm that utilizes an active site mutant D134A (D3.32) 5-HT2C receptor to identify atypical agonist structures. We additionally report the discovery and optimization of a novel class of nonbasic heterocyclic amide agonists of 5-HT2C. SAR investigations around the screening hits provided a diverse set of potent agonists at 5-HT2C with high selectivity over the related 5-HT2A and 5-HT2B receptor subtypes. Further optimization through replacement of the amide with a variety of five- and six-membered heterocycles led to the identification of 6-(1-ethyl-3-(quinolin-8-yl)-1H-pyrazol-5-yl)pyridazin-3-amine (69). Oral administration of 69 to rats reduced food intake in an ad libitum feeding model, which could be completely reversed by a selective 5-HT2C antagonist.
中文翻译:

利用活性位点突变受体鉴定有效的和选择性的非典型5-HT 2C受体激动剂。
5-HT 2C受体的拮抗作用是药理学减轻体重的最深入研究和临床证明的机制之一。对密切相关的5-HT 2A和5-HT 2B受体的选择性至关重要,因为已证明它们的活化会导致不良的副作用和重大的安全隐患。在此通讯中,我们报告了一种新的筛选范例的开发,该范例利用了活性位点突变体D134A(D3.32)5-HT 2C受体来鉴定非典型激动剂结构。我们还报告了5-HT 2C的新型非碱性杂环酰胺激动剂的发现和优化。围绕筛选结果的SAR调查为5-HT提供了多种有效的激动剂2C具有对相关5-HT 2A和5-HT 2B受体亚型的高选择性。通过用各种五元和六元杂环取代酰胺进行进一步优化,导致鉴定出6-(1-乙基-3-(喹啉-8-基)-1 H-吡唑-5-基)哒嗪-3-胺(69)。老鼠口服给药69可减少随意采食模型中的食物摄入量,而选择性5-HT 2C拮抗剂可完全逆转这种情况。
更新日期:2017-07-08
中文翻译:

利用活性位点突变受体鉴定有效的和选择性的非典型5-HT 2C受体激动剂。
5-HT 2C受体的拮抗作用是药理学减轻体重的最深入研究和临床证明的机制之一。对密切相关的5-HT 2A和5-HT 2B受体的选择性至关重要,因为已证明它们的活化会导致不良的副作用和重大的安全隐患。在此通讯中,我们报告了一种新的筛选范例的开发,该范例利用了活性位点突变体D134A(D3.32)5-HT 2C受体来鉴定非典型激动剂结构。我们还报告了5-HT 2C的新型非碱性杂环酰胺激动剂的发现和优化。围绕筛选结果的SAR调查为5-HT提供了多种有效的激动剂2C具有对相关5-HT 2A和5-HT 2B受体亚型的高选择性。通过用各种五元和六元杂环取代酰胺进行进一步优化,导致鉴定出6-(1-乙基-3-(喹啉-8-基)-1 H-吡唑-5-基)哒嗪-3-胺(69)。老鼠口服给药69可减少随意采食模型中的食物摄入量,而选择性5-HT 2C拮抗剂可完全逆转这种情况。



























 京公网安备 11010802027423号
京公网安备 11010802027423号