当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Self-Assembled Framework Formed During Lithiation of SnS2 Nanoplates Revealed by in Situ Electron Microscopy
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-07-06 00:00:00 , DOI: 10.1021/acs.accounts.7b00086 Kuibo Yin 1, 2 , Minghao Zhang 2, 3 , Zachary D. Hood 2, 4 , Jie Pan 5 , Ying Shirley Meng 3 , Miaofang Chi 2
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-07-06 00:00:00 , DOI: 10.1021/acs.accounts.7b00086 Kuibo Yin 1, 2 , Minghao Zhang 2, 3 , Zachary D. Hood 2, 4 , Jie Pan 5 , Ying Shirley Meng 3 , Miaofang Chi 2
Affiliation
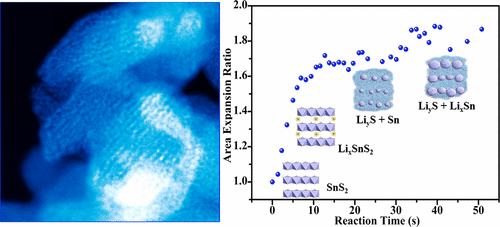
|
Lithium-ion batteries (LIBs) commercially dominate portable energy storage and have been extended to hybrid/electric vehicles by utilizing electrode materials with enhanced energy density. However, the energy density and cycling life of LIBs must extend beyond the current reach of commercial electrodes to meet the performance requirements for transportation applications. Carbon-based anodes, serving as the main negative electrodes in LIBs, have an intrinsic capacity limitation due to the intercalation mechanism. Some nanostructured carbon materials offer very interesting reversible capacities and can be considered as future anode materials. However, their fabrication processes are often complicated and expensive. Theoretically, using a lithium metal anode is the best way of delivering high energy density due to its largest theoretical capacity of more than 3800 mAh g–1; however, lithium metal is highly reactive with liquid electrolytes. Alternative anodes are being explored, including other lithium-reactive metals, such as Si, Ge, Zn, V, and so forth. These metals react reversibly with a large amount of Li per formula unit to form lithium–metal alloys, rendering these materials promising candidates for next-generation LIBs with high energy density. Though, most of these pure metallic anodes experience large volume changes during lithiation and delithiation processes that often results in cracking of the anode material and a loss electrical contact between the particles.
中文翻译:

原位电子显微镜揭示SnS 2纳米板的锂化过程中形成的自组装框架。
锂离子电池(LIB)在商业上主导了便携式储能,并已通过利用具有增强的能量密度的电极材料扩展到了混合动力/电动汽车。但是,LIB的能量密度和循环寿命必须超出商用电极的电流范围,才能满足运输应用的性能要求。碳基阳极作为LIB中的主要负极,由于插层机理而具有固有的容量限制。一些纳米结构的碳材料提供了非常有趣的可逆容量,可以被认为是未来的阳极材料。然而,它们的制造过程通常是复杂且昂贵的。从理论上讲–1 ; 然而,锂金属与液体电解质高度反应。正在探索替代阳极,包括其他锂反应性金属,例如Si,Ge,Zn,V等。这些金属与每个配方单元中的大量Li可逆反应形成锂金属合金,使这些材料有望成为具有高能量密度的下一代LIB的候选材料。但是,大多数这些纯金属阳极在锂化和脱锂过程中会经历较大的体积变化,这通常会导致阳极材料开裂和颗粒之间失去电接触。
更新日期:2017-07-06
中文翻译:

原位电子显微镜揭示SnS 2纳米板的锂化过程中形成的自组装框架。
锂离子电池(LIB)在商业上主导了便携式储能,并已通过利用具有增强的能量密度的电极材料扩展到了混合动力/电动汽车。但是,LIB的能量密度和循环寿命必须超出商用电极的电流范围,才能满足运输应用的性能要求。碳基阳极作为LIB中的主要负极,由于插层机理而具有固有的容量限制。一些纳米结构的碳材料提供了非常有趣的可逆容量,可以被认为是未来的阳极材料。然而,它们的制造过程通常是复杂且昂贵的。从理论上讲–1 ; 然而,锂金属与液体电解质高度反应。正在探索替代阳极,包括其他锂反应性金属,例如Si,Ge,Zn,V等。这些金属与每个配方单元中的大量Li可逆反应形成锂金属合金,使这些材料有望成为具有高能量密度的下一代LIB的候选材料。但是,大多数这些纯金属阳极在锂化和脱锂过程中会经历较大的体积变化,这通常会导致阳极材料开裂和颗粒之间失去电接触。



























 京公网安备 11010802027423号
京公网安备 11010802027423号