当前位置:
X-MOL 学术
›
Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Characteristics of methylammonium ion (CH3NH3+) in aqueous electrolyte solution: An ONIOM-XS MD simulation study
Chemical Physics ( IF 2.3 ) Pub Date : 2017-06-27 00:00:00 , DOI: 10.1016/j.chemphys.2017.06.012 Prangthong Chaiyasit , Anan Tongraar , Teerakiat Kerdcharoen
Chemical Physics ( IF 2.3 ) Pub Date : 2017-06-27 00:00:00 , DOI: 10.1016/j.chemphys.2017.06.012 Prangthong Chaiyasit , Anan Tongraar , Teerakiat Kerdcharoen
|
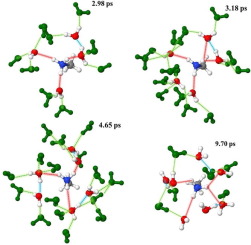
An ONIOM-XS MD simulation has been performed to characterize the CH3NH3+-water hydrogen bonds (HBs) in aqueous solution. The sphere which includes the CH3NH3+ ion and its surrounding waters was treated by the HF/DZP method, while the rest was described by classical pair potentials. The ONIOM-XS MD results clearly reveal a flexible CH3NH3+ solvation, showing various numbers of water molecules, ranging from 3 to 8 and from 12 to 19, cooperatively involved in the primary region of the -NH3+ and -CH3 species, respectively. The –NH3+ group participates in about 3.6 HBs with its nearest-neighbor waters, and the HBs between the –NH3+ hydrogens and their nearest-neighbor waters are relatively weaker than the HBs of bulk water. It is evident that the “hydrophobic effect” of the –CH3 species results in slightly more attractive water-water HB interactions in this region. Such phenomenon corresponds to a clear “structure-breaking” ability of CH3NH3+ in aqueous solution.
中文翻译:

电解质水溶液中甲基铵离子(CH 3 NH 3 +)的特性:ONIOM-XS MD模拟研究
已经进行了ONIOM-XS MD模拟,以表征水溶液中的CH 3 NH 3 +-水氢键(HBs)。通过HF / DZP方法处理了包含CH 3 NH 3 +离子及其周围水域的球体,而其余部分则通过经典对势进行了描述。ONIOM-XS MD结果清楚地揭示了一种灵活的CH 3 NH 3 +溶剂化作用,表明在-NH 3 +和-CH的主要区域中协同参与了3至8个和12至19个不同数量的水分子分别为3种。–NH 3 +该组成员与其近邻水域共参与约3.6 HBs,–NH 3 +氢与其近邻水域之间的HBs相对弱于散水的HBs。显然,–CH 3物种的“疏水作用”导致该区域水-水HB相互作用更具吸引力。这种现象对应于水溶液中CH 3 NH 3 +的明显的“破坏结构”能力。
更新日期:2017-06-28
中文翻译:

电解质水溶液中甲基铵离子(CH 3 NH 3 +)的特性:ONIOM-XS MD模拟研究
已经进行了ONIOM-XS MD模拟,以表征水溶液中的CH 3 NH 3 +-水氢键(HBs)。通过HF / DZP方法处理了包含CH 3 NH 3 +离子及其周围水域的球体,而其余部分则通过经典对势进行了描述。ONIOM-XS MD结果清楚地揭示了一种灵活的CH 3 NH 3 +溶剂化作用,表明在-NH 3 +和-CH的主要区域中协同参与了3至8个和12至19个不同数量的水分子分别为3种。–NH 3 +该组成员与其近邻水域共参与约3.6 HBs,–NH 3 +氢与其近邻水域之间的HBs相对弱于散水的HBs。显然,–CH 3物种的“疏水作用”导致该区域水-水HB相互作用更具吸引力。这种现象对应于水溶液中CH 3 NH 3 +的明显的“破坏结构”能力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号