当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
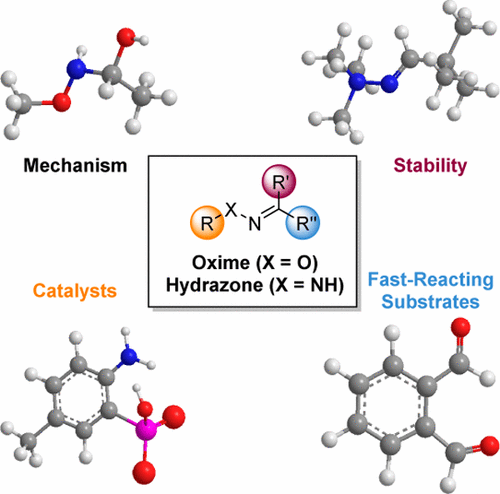
Oximes and Hydrazones in Bioconjugation: Mechanism and Catalysis
Chemical Reviews ( IF 62.1 ) Pub Date : 2017-06-22 00:00:00 , DOI: 10.1021/acs.chemrev.7b00090 Dominik K. Kölmel 1 , Eric T. Kool 1
Chemical Reviews ( IF 62.1 ) Pub Date : 2017-06-22 00:00:00 , DOI: 10.1021/acs.chemrev.7b00090 Dominik K. Kölmel 1 , Eric T. Kool 1
Affiliation
|
The formation of oximes and hydrazones is employed in numerous scientific fields as a simple and versatile conjugation strategy. This imine-forming reaction is applied in fields as diverse as polymer chemistry, biomaterials and hydrogels, dynamic combinatorial chemistry, organic synthesis, and chemical biology. Here we outline chemical developments in this field, with special focus on the past ∼10 years of developments. Recent strategies for installing reactive carbonyl groups and α-nucleophiles into biomolecules are described. The basic chemical properties of reactants and products in this reaction are then reviewed, with an eye to understanding the reaction’s mechanism and how reactant structure controls rates and equilibria in the process. Recent work that has uncovered structural features and new mechanisms for speeding the reaction, sometimes by orders of magnitude, is discussed. We describe recent studies that have identified especially fast reacting aldehyde/ketone substrates and structural effects that lead to rapid-reacting α-nucleophiles as well. Among the most effective new strategies has been the development of substituents near the reactive aldehyde group that either transfer protons at the transition state or trap the initially formed tetrahedral intermediates. In addition, the recent development of efficient nucleophilic catalysts for the reaction is outlined, improving greatly upon aniline, the classical catalyst for imine formation. A number of uses of such second- and third-generation catalysts in bioconjugation and in cellular applications are highlighted. While formation of hydrazone and oxime has been traditionally regarded as being limited by slow rates, developments in the past 5 years have resulted in completely overturning this limitation; indeed, the reaction is now one of the fastest and most versatile reactions available for conjugations of biomolecules and biomaterials.
中文翻译:

生物缀合中的肟和Hy:机理和催化作用
肟和的形成在许多科学领域中被用作一种简单而通用的结合策略。这种形成亚胺的反应广泛应用于聚合物化学,生物材料和水凝胶,动态组合化学,有机合成和化学生物学等领域。在这里,我们概述了该领域的化学发展,特别着眼于过去约10年的发展。描述了将反应性羰基和α-亲核试剂安装到生物分子中的最新策略。然后回顾了该反应中反应物和产物的基本化学性质,以期了解反应的机理以及反应物结构如何控制过程中的速率和平衡。最近的工作发现了加快反应的结构特征和新机制,有时会被数量级地讨论。我们描述了最近的研究,这些研究已经确定了特别快速反应的醛/酮底物和导致快速反应的α-亲核试剂的结构效应。最有效的新策略之一是在反应性醛基附近形成取代基,这些取代基要么在过渡态转移质子,要么捕获最初形成的四面体中间体。此外,概述了该反应的有效亲核催化剂的最新进展,大大改进了苯胺(亚胺形成的经典催化剂)。强调了这种第二代和第三代催化剂在生物缀合和细胞应用中的许多用途。传统上of和肟的形成受到缓慢速率的限制,过去5年的发展已完全推翻了这一局限性;实际上,该反应现在是可用于结合生物分子和生物材料的最快,最通用的反应之一。
更新日期:2017-06-22
中文翻译:

生物缀合中的肟和Hy:机理和催化作用
肟和的形成在许多科学领域中被用作一种简单而通用的结合策略。这种形成亚胺的反应广泛应用于聚合物化学,生物材料和水凝胶,动态组合化学,有机合成和化学生物学等领域。在这里,我们概述了该领域的化学发展,特别着眼于过去约10年的发展。描述了将反应性羰基和α-亲核试剂安装到生物分子中的最新策略。然后回顾了该反应中反应物和产物的基本化学性质,以期了解反应的机理以及反应物结构如何控制过程中的速率和平衡。最近的工作发现了加快反应的结构特征和新机制,有时会被数量级地讨论。我们描述了最近的研究,这些研究已经确定了特别快速反应的醛/酮底物和导致快速反应的α-亲核试剂的结构效应。最有效的新策略之一是在反应性醛基附近形成取代基,这些取代基要么在过渡态转移质子,要么捕获最初形成的四面体中间体。此外,概述了该反应的有效亲核催化剂的最新进展,大大改进了苯胺(亚胺形成的经典催化剂)。强调了这种第二代和第三代催化剂在生物缀合和细胞应用中的许多用途。传统上of和肟的形成受到缓慢速率的限制,过去5年的发展已完全推翻了这一局限性;实际上,该反应现在是可用于结合生物分子和生物材料的最快,最通用的反应之一。


























 京公网安备 11010802027423号
京公网安备 11010802027423号