当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric Synthesis of Butenolides and Butyrolactones
Chemical Reviews ( IF 62.1 ) Pub Date : 2017-06-22 00:00:00 , DOI: 10.1021/acs.chemrev.7b00151 Bin Mao 1, 2 , Martín Fañanás-Mastral 1, 3 , Ben L. Feringa 1
Chemical Reviews ( IF 62.1 ) Pub Date : 2017-06-22 00:00:00 , DOI: 10.1021/acs.chemrev.7b00151 Bin Mao 1, 2 , Martín Fañanás-Mastral 1, 3 , Ben L. Feringa 1
Affiliation
|
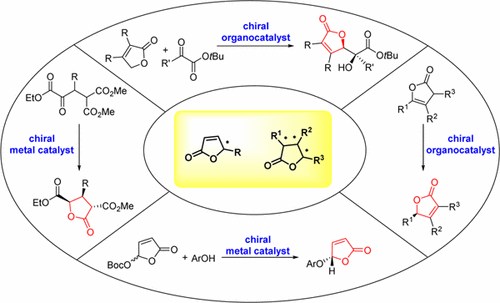
γ-Butenolides, γ-butyrolactones, and derivatives, especially in enantiomerically pure form, constitute the structural core of numerous natural products which display an impressive range of biological activities which are important for the development of novel physiological and therapeutic agents. Furthermore, optically active γ-butenolides and γ-butyrolactones serve also as a prominent class of chiral building blocks for the synthesis of diverse biological active compounds and complex molecules. Taking into account the varying biological activity profiles and wide-ranging structural diversity of the optically active γ-butenolide or γ-butyrolactone structure, the development of asymmetric synthetic strategies for assembling such challenging scaffolds has attracted major attention from synthetic chemists in the past decade. This review offers an overview of the different enantioselective synthesis of γ-butenolides and γ-butyrolactones which employ catalytic amounts of metal complexes or organocatalysts, with emphasis focused on the mechanistic issues that account for the observed stereocontrol of the representative reactions, as well as practical applications and synthetic potentials.
中文翻译:

丁烯内酯和丁内酯的催化不对称合成
γ-丁烯内酯,γ-丁内酯及其衍生物,特别是对映体纯形式,构成许多天然产物的结构核心,这些天然产物表现出令人印象深刻的生物学活性,这对于开发新型生理学和治疗剂至关重要。此外,旋光性γ-丁烯内酯和γ-丁内酯类也是合成各种生物活性化合物和复杂分子的主要手性结构单元。考虑到旋光性γ-丁烯内酯或γ-丁内酯结构的不同生物活性谱和广泛的结构多样性,在过去十年中,组装此类具有挑战性的支架的不对称合成策略的发展引起了合成化学家的主要关注。
更新日期:2017-06-22
中文翻译:

丁烯内酯和丁内酯的催化不对称合成
γ-丁烯内酯,γ-丁内酯及其衍生物,特别是对映体纯形式,构成许多天然产物的结构核心,这些天然产物表现出令人印象深刻的生物学活性,这对于开发新型生理学和治疗剂至关重要。此外,旋光性γ-丁烯内酯和γ-丁内酯类也是合成各种生物活性化合物和复杂分子的主要手性结构单元。考虑到旋光性γ-丁烯内酯或γ-丁内酯结构的不同生物活性谱和广泛的结构多样性,在过去十年中,组装此类具有挑战性的支架的不对称合成策略的发展引起了合成化学家的主要关注。



























 京公网安备 11010802027423号
京公网安备 11010802027423号