当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Halofunctionalization of alkenes by vanadium chloroperoxidase from Curvularia inaequalis
Chemical Communications ( IF 4.9 ) Pub Date : 2017-05-22 00:00:00 , DOI: 10.1039/c7cc03368k Jia Jia Dong 1, 2, 3, 4 , Elena Fernández-Fueyo 1, 2, 3, 4 , Jingbo Li 5, 6, 7, 8 , Zheng Guo 5, 6, 7, 8 , Rokus Renirie 4, 9, 10, 11, 12 , Ron Wever 4, 9, 10, 11, 12 , Frank Hollmann 1, 2, 3, 4
Chemical Communications ( IF 4.9 ) Pub Date : 2017-05-22 00:00:00 , DOI: 10.1039/c7cc03368k Jia Jia Dong 1, 2, 3, 4 , Elena Fernández-Fueyo 1, 2, 3, 4 , Jingbo Li 5, 6, 7, 8 , Zheng Guo 5, 6, 7, 8 , Rokus Renirie 4, 9, 10, 11, 12 , Ron Wever 4, 9, 10, 11, 12 , Frank Hollmann 1, 2, 3, 4
Affiliation
|
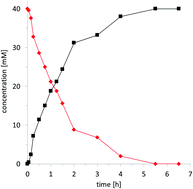
The vanadium-dependent chloroperoxidase from Curvularia inaequalis is a stable and efficient biocatalyst for the hydroxyhalogenation of a broad range of alkenes into halohydrins. Up to 1 200
200 000 TON with 69 s−1 TOF were observed for the biocatalyst. A bienzymatic cascade to yield epoxides as reaction products is presented.
000 TON with 69 s−1 TOF were observed for the biocatalyst. A bienzymatic cascade to yield epoxides as reaction products is presented.
中文翻译:

从钒氯代烯烃Halofunctionalization不等弯孢霉
来自弯孢不动杆菌的钒依赖性氯过氧化物酶是一种稳定有效的生物催化剂,用于将多种烯烃羟基卤化为卤代醇。对于该生物催化剂,观察到高达 1200
1200 万吨的TOS为69 s -1。提出了双酶级联反应以产生环氧化物作为反应产物。
万吨的TOS为69 s -1。提出了双酶级联反应以产生环氧化物作为反应产物。
更新日期:2017-05-27
 200
200 000 TON with 69 s−1 TOF were observed for the biocatalyst. A bienzymatic cascade to yield epoxides as reaction products is presented.
000 TON with 69 s−1 TOF were observed for the biocatalyst. A bienzymatic cascade to yield epoxides as reaction products is presented.
中文翻译:

从钒氯代烯烃Halofunctionalization不等弯孢霉
来自弯孢不动杆菌的钒依赖性氯过氧化物酶是一种稳定有效的生物催化剂,用于将多种烯烃羟基卤化为卤代醇。对于该生物催化剂,观察到高达
 1200
1200 万吨的TOS为69 s -1。提出了双酶级联反应以产生环氧化物作为反应产物。
万吨的TOS为69 s -1。提出了双酶级联反应以产生环氧化物作为反应产物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号