当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total Synthesis of (±)-Corymine
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2017-05-23 03:41:06 , DOI: 10.1002/anie.201704086 Benxiang Zhang 1 , Xiaoqing Wang 1 , Chao Cheng 1 , Deqian Sun 1 , Chaozhong Li 1, 2
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2017-05-23 03:41:06 , DOI: 10.1002/anie.201704086 Benxiang Zhang 1 , Xiaoqing Wang 1 , Chao Cheng 1 , Deqian Sun 1 , Chaozhong Li 1, 2
Affiliation
|
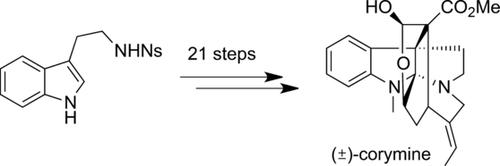
The first total synthesis of the hexacyclic indole alkaloid (±)-corymine is described. Starting from the readily available N-protected tryptamine, the title compound was achieved in 21 steps in 3.4 % overall yield. Key steps of the synthesis include: a) the addition of a malonate to a 3-bromooxindole to afford 3,3-disubstituted oxindole, b) the formation of a 12-membered cyclic enol ether by intramolecular O-propargylation, immediately followed by propargyl Claisen rearrangement to provide the α-allenyl ketone stereospecifically, c) DMDO oxidation to install a hydroxy group in a highly stereoselective manner, and d) the SmI2-mediated reductive C−O bond cleavage to remove the α-keto carboxyl group.
中文翻译:

(±)-甘氨酸的全合成
描述了六环吲哚生物碱(±)-胭脂红的第一全合成。从容易获得的N-保护的色胺开始,以21个步骤获得标题化合物,总产率为3.4%。合成的关键步骤包括:a)将丙二酸酯添加到3-溴代吲哚中,得到3,3-二取代的羟吲哚,b)通过分子内O-炔丙基化,立即接着炔丙基形成12元环烯醇醚克莱森重排以立体定向地提供α-烯丙基酮,c)DMDO氧化以高度立体选择性的方式安装羟基,d)SmI 2介导的还原性C-O键裂解以去除α-酮基羧基。
更新日期:2017-05-24
中文翻译:

(±)-甘氨酸的全合成
描述了六环吲哚生物碱(±)-胭脂红的第一全合成。从容易获得的N-保护的色胺开始,以21个步骤获得标题化合物,总产率为3.4%。合成的关键步骤包括:a)将丙二酸酯添加到3-溴代吲哚中,得到3,3-二取代的羟吲哚,b)通过分子内O-炔丙基化,立即接着炔丙基形成12元环烯醇醚克莱森重排以立体定向地提供α-烯丙基酮,c)DMDO氧化以高度立体选择性的方式安装羟基,d)SmI 2介导的还原性C-O键裂解以去除α-酮基羧基。


























 京公网安备 11010802027423号
京公网安备 11010802027423号