当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biocatalytic Friedel-Crafts Acylation and Fries Reaction.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2017-05-23 , DOI: 10.1002/anie.201703270 Nina G Schmidt 1, 2 , Tea Pavkov-Keller 1, 3 , Nina Richter 1, 2 , Birgit Wiltschi 1 , Karl Gruber 1, 3 , Wolfgang Kroutil 1, 2
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2017-05-23 , DOI: 10.1002/anie.201703270 Nina G Schmidt 1, 2 , Tea Pavkov-Keller 1, 3 , Nina Richter 1, 2 , Birgit Wiltschi 1 , Karl Gruber 1, 3 , Wolfgang Kroutil 1, 2
Affiliation
|
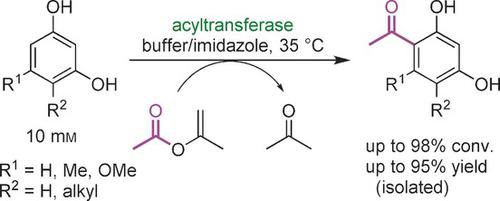
The Friedel-Crafts acylation is commonly used for the synthesis of aryl ketones, and a biocatalytic version, which may benefit from the chemo- and regioselectivity of enzymes, has not yet been introduced. Described here is a bacterial acyltransferase which can catalyze Friedel-Crafts C-acylation of phenolic substrates in buffer without the need of CoA-activated reagents. Conversions reach up to >99 %, and various C- or O-acyl donors, such as DAPG or isopropenyl acetate, are accepted by this enzyme. Furthermore the enzyme enables a Fries rearrangement-like reaction of resorcinol derivatives. These findings open an avenue for the development of alternative and selective C-C bond formation methods.
中文翻译:

生物催化弗里德尔-克来福特酰化和弗里斯反应。
弗里德尔-克来福特酰化通常用于芳基酮的合成,并且尚未引入可能受益于酶的化学和区域选择性的生物催化版本。这里描述的是一种细菌酰基转移酶,它可以催化缓冲液中酚类底物的 Friedel-Crafts C-酰化,而不需要 CoA 激活试剂。转化率高达 >99%,并且该酶接受各种 C-或 O-酰基供体,例如 DAPG 或乙酸异丙烯酯。此外,该酶能够使间苯二酚衍生物发生类似弗里斯重排的反应。这些发现为开发替代性和选择性 CC 键形成方法开辟了道路。
更新日期:2017-05-23
中文翻译:

生物催化弗里德尔-克来福特酰化和弗里斯反应。
弗里德尔-克来福特酰化通常用于芳基酮的合成,并且尚未引入可能受益于酶的化学和区域选择性的生物催化版本。这里描述的是一种细菌酰基转移酶,它可以催化缓冲液中酚类底物的 Friedel-Crafts C-酰化,而不需要 CoA 激活试剂。转化率高达 >99%,并且该酶接受各种 C-或 O-酰基供体,例如 DAPG 或乙酸异丙烯酯。此外,该酶能够使间苯二酚衍生物发生类似弗里斯重排的反应。这些发现为开发替代性和选择性 CC 键形成方法开辟了道路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号