当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct Nucleophilic Substitution Reaction of Cage B−H Bonds by Grignard Reagents: A Route to Regioselective B4‐Alkylation of o‐Carboranes
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2017-05-23 , DOI: 10.1002/anie.201702347 Cen Tang 1 , Jiji Zhang 1 , Zuowei Xie 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2017-05-23 , DOI: 10.1002/anie.201702347 Cen Tang 1 , Jiji Zhang 1 , Zuowei Xie 1
Affiliation
|
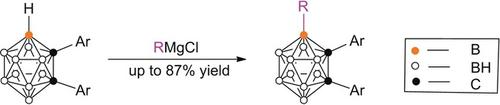
Direct nucleophilic substitution reaction of cage B−H bonds of o‐carboranes by Grignard reagents in the absence of any transition metals has been achieved for the first time, and leads to the regioselective synthesis of a series of 4‐alkyl‐1,2‐diaryl‐o‐carboranes in very high yields. The presence of two electron‐withdrawing aryl groups on the cage carbon atoms is crucial to realizing the reaction. The regioselectivity is controlled by both electronic and steric factors. This work represents a new strategy for the development of methods for carborane functionalization.
中文翻译:

格氏试剂对笼型B-H键的直接亲核取代反应:邻位硼烷的区域选择性B4烷基化的途径
在没有任何过渡金属的情况下,首次实现了格氏试剂在邻氨基甲酸酯的笼状B-H键之间的直接亲核取代反应,并导致了一系列4烷基1,2-烷基的区域选择性合成二芳基Ø -carboranes在非常高的产量。笼状碳原子上存在两个吸电子芳基对于实现该反应至关重要。区域选择性受电子和空间因素控制。这项工作代表了开发碳硼烷功能化方法的新策略。
更新日期:2017-05-23
中文翻译:

格氏试剂对笼型B-H键的直接亲核取代反应:邻位硼烷的区域选择性B4烷基化的途径
在没有任何过渡金属的情况下,首次实现了格氏试剂在邻氨基甲酸酯的笼状B-H键之间的直接亲核取代反应,并导致了一系列4烷基1,2-烷基的区域选择性合成二芳基Ø -carboranes在非常高的产量。笼状碳原子上存在两个吸电子芳基对于实现该反应至关重要。区域选择性受电子和空间因素控制。这项工作代表了开发碳硼烷功能化方法的新策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号