当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanisms and Origins of Chemo- and Regioselectivities of Ru(II)-Catalyzed Decarboxylative C–H Alkenylation of Aryl Carboxylic Acids with Alkynes: A Computational Study
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-05-12 00:00:00 , DOI: 10.1021/jacs.7b00714 Jing-Lu Yu 1 , Shuo-Qing Zhang 1 , Xin Hong 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-05-12 00:00:00 , DOI: 10.1021/jacs.7b00714 Jing-Lu Yu 1 , Shuo-Qing Zhang 1 , Xin Hong 1
Affiliation
|
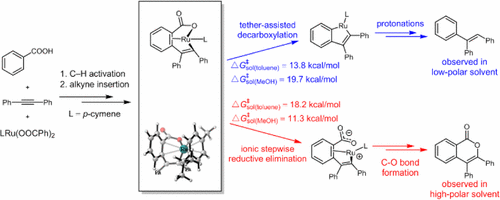
The mechanisms and chemo- and regioselectivities of Ru(II)-catalyzed decarboxylative C–H alkenylation of aryl carboxylic acids with alkynes were investigated with density functional theory (DFT) calculations. The catalytic cycle involves sequential carboxylate-directed C–H activation, alkyne insertion, decarboxylation and protonation. The facile tether-assisted decarboxylation step directs the intermediate toward the desired decarboxylative alkenylation, instead of typical annulation and double alkenylation pathways. The decarboxylation barrier is very sensitive to the tether length, and only the seven-membered ring intermediate can selectively undergo the designed decarboxylation, suggesting a tether-dependent chemoselectivity. This tether-dependent chemoselectivity also applies to the alkyl tethers. In addition, the polarity of solvent is found to control the chemoselectivity between the decarboxylative alkenylation and [4 + 2] annulation. Solvent with low polarity (toluene) favors the decarboxylation pathway, leading to the decarboxylative alkenylation. Solvent with high polarity (methanol) favors the ionic stepwise C–O reductive elimination pathway, leading to the [4 + 2] annulation. To understand the origins of regioselectivity with asymmetric alkynes, the distortion/interaction analysis was applied to the alkyne insertion transition states, and led to a predictive frontier molecular orbital model. The asymmetric alkynes selectively use the terminal with the larger HOMO orbital coefficient to form the C–C bond in the insertion step.
中文翻译:

Ru(II)催化炔烃与炔烃的脱羧CH-H烯基化反应的化学和区域选择性的机理和起源:计算研究
用密度泛函理论(DFT)计算研究了Ru(II)催化炔烃芳基羧酸脱羧CH烯基化反应的机理,化学和区域选择性。催化循环涉及顺序的羧酸盐定向的C–H活化,炔烃的插入,脱羧和质子化。简便的系链辅助脱羧步骤将中间体导向所需的脱羧烯基化,而不是典型的环化和双烯基化途径。脱羧屏障对系链长度非常敏感,并且只有七元环中间体可以选择性地进行设计的脱羧,这表明了系链依赖性的化学选择性。这种依赖于系链的化学选择性也适用于烷基系链。此外,发现溶剂的极性可控制脱羧烯基化与[4 + 2]环化反应之间的化学选择性。低极性溶剂(甲苯)有利于脱羧途径,导致脱羧烯基化。具有高极性的溶剂(甲醇)有利于离子逐步C–O还原消除途径,从而导致[4 + 2]环化。为了了解不对称炔烃的区域选择性的起源,将畸变/相互作用分析应用于炔烃插入过渡态,并建立了预测性前沿分子轨道模型。非对称炔烃在插入步骤中选择性使用具有较高HOMO轨道系数的末端形成C–C键。低极性溶剂(甲苯)有利于脱羧途径,导致脱羧烯基化。具有高极性的溶剂(甲醇)有利于离子逐步C–O还原消除途径,从而导致[4 + 2]环化。为了了解不对称炔烃的区域选择性的起源,将畸变/相互作用分析应用于炔烃插入过渡态,并建立了预测性前沿分子轨道模型。非对称炔烃在插入步骤中选择性使用具有较高HOMO轨道系数的末端形成C–C键。低极性溶剂(甲苯)有利于脱羧途径,导致脱羧烯基化。具有高极性的溶剂(甲醇)有利于离子逐步C–O还原消除途径,从而导致[4 + 2]环化。为了了解不对称炔烃的区域选择性的起源,将畸变/相互作用分析应用于炔烃插入过渡态,并建立了预测性前沿分子轨道模型。非对称炔烃在插入步骤中选择性使用具有较高HOMO轨道系数的末端形成C–C键。为了了解不对称炔烃的区域选择性的起源,将畸变/相互作用分析应用于炔烃插入过渡态,并建立了预测性前沿分子轨道模型。非对称炔烃在插入步骤中选择性使用具有较高HOMO轨道系数的末端形成C–C键。为了了解不对称炔烃的区域选择性的起源,将畸变/相互作用分析应用于炔烃插入过渡态,并建立了预测性前沿分子轨道模型。非对称炔烃在插入步骤中选择性使用具有较高HOMO轨道系数的末端形成C–C键。
更新日期:2017-05-23
中文翻译:

Ru(II)催化炔烃与炔烃的脱羧CH-H烯基化反应的化学和区域选择性的机理和起源:计算研究
用密度泛函理论(DFT)计算研究了Ru(II)催化炔烃芳基羧酸脱羧CH烯基化反应的机理,化学和区域选择性。催化循环涉及顺序的羧酸盐定向的C–H活化,炔烃的插入,脱羧和质子化。简便的系链辅助脱羧步骤将中间体导向所需的脱羧烯基化,而不是典型的环化和双烯基化途径。脱羧屏障对系链长度非常敏感,并且只有七元环中间体可以选择性地进行设计的脱羧,这表明了系链依赖性的化学选择性。这种依赖于系链的化学选择性也适用于烷基系链。此外,发现溶剂的极性可控制脱羧烯基化与[4 + 2]环化反应之间的化学选择性。低极性溶剂(甲苯)有利于脱羧途径,导致脱羧烯基化。具有高极性的溶剂(甲醇)有利于离子逐步C–O还原消除途径,从而导致[4 + 2]环化。为了了解不对称炔烃的区域选择性的起源,将畸变/相互作用分析应用于炔烃插入过渡态,并建立了预测性前沿分子轨道模型。非对称炔烃在插入步骤中选择性使用具有较高HOMO轨道系数的末端形成C–C键。低极性溶剂(甲苯)有利于脱羧途径,导致脱羧烯基化。具有高极性的溶剂(甲醇)有利于离子逐步C–O还原消除途径,从而导致[4 + 2]环化。为了了解不对称炔烃的区域选择性的起源,将畸变/相互作用分析应用于炔烃插入过渡态,并建立了预测性前沿分子轨道模型。非对称炔烃在插入步骤中选择性使用具有较高HOMO轨道系数的末端形成C–C键。低极性溶剂(甲苯)有利于脱羧途径,导致脱羧烯基化。具有高极性的溶剂(甲醇)有利于离子逐步C–O还原消除途径,从而导致[4 + 2]环化。为了了解不对称炔烃的区域选择性的起源,将畸变/相互作用分析应用于炔烃插入过渡态,并建立了预测性前沿分子轨道模型。非对称炔烃在插入步骤中选择性使用具有较高HOMO轨道系数的末端形成C–C键。为了了解不对称炔烃的区域选择性的起源,将畸变/相互作用分析应用于炔烃插入过渡态,并建立了预测性前沿分子轨道模型。非对称炔烃在插入步骤中选择性使用具有较高HOMO轨道系数的末端形成C–C键。为了了解不对称炔烃的区域选择性的起源,将畸变/相互作用分析应用于炔烃插入过渡态,并建立了预测性前沿分子轨道模型。非对称炔烃在插入步骤中选择性使用具有较高HOMO轨道系数的末端形成C–C键。



























 京公网安备 11010802027423号
京公网安备 11010802027423号