当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total Chemical Synthesis and Folding of All-l and All-d Variants of Oncogenic KRas(G12V)
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-04-27 00:00:00 , DOI: 10.1021/jacs.7b02988 Adam M. Levinson 1 , John H. McGee 2 , Andrew G. Roberts , Gardner S. Creech , Ting Wang , Michael T. Peterson , Ronald C. Hendrickson , Gregory L. Verdine 2 , Samuel J. Danishefsky 3
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-04-27 00:00:00 , DOI: 10.1021/jacs.7b02988 Adam M. Levinson 1 , John H. McGee 2 , Andrew G. Roberts , Gardner S. Creech , Ting Wang , Michael T. Peterson , Ronald C. Hendrickson , Gregory L. Verdine 2 , Samuel J. Danishefsky 3
Affiliation
|
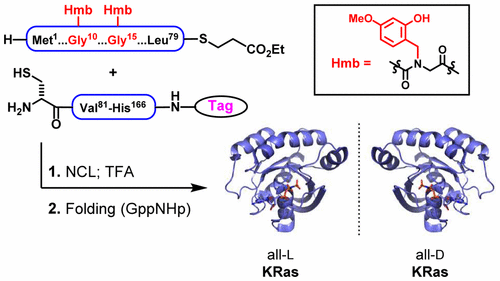
The Ras proteins are essential GTPases involved in the regulation of cell proliferation and survival. Mutated oncogenic forms of Ras alter effector binding and innate GTPase activity, leading to deregulation of downstream signal transduction. Mutated forms of Ras are involved in approximately 30% of human cancers. Despite decades of effort to develop direct Ras inhibitors, Ras has long been considered “undruggable” due to its high affinity for GTP and its lack of hydrophobic binding pockets. Herein, we report a total chemical synthesis of all-l- and all-d-amino acid biotinylated variants of oncogenic mutant KRas(G12V). The protein is synthesized using Fmoc-based solid-phase peptide synthesis and assembled using combined native chemical ligation and isonitrile-mediated activation strategies. We demonstrate that both KRas(G12V) enantiomers can successfully fold and bind nucleotide substrates and binding partners with observable enantiodiscrimination. By demonstrating the functional competency of a mirror-image form of KRas bound to its corresponding enantiomeric nucleotide triphosphate, this study sets the stage for further biochemical studies with this material. In particular, this protein will enable mirror-image yeast surface display experiments to identify all-d peptide ligands for oncogenic KRas, providing a useful tool in the search for new therapeutics against this challenging disease target.
中文翻译:

致癌性KRas(G12V) All- 1和All- d变体的总化学合成和折叠
Ras蛋白是参与细胞增殖和存活调节的必需的GTPases。Ras的致癌突变形式会改变效应子结合和固有的GTPase活性,从而导致下游信号转导的失调。Ras的突变形式涉及大约30%的人类癌症。尽管数十年来一直在努力开发直接的Ras抑制剂,但由于Ras对GTP的亲和力高以及缺乏疏水性结合口袋,长期以来一直被认为是“不可药物的”。在这里,我们报告清一色的全合成升-和清一色d-致癌突变体KRas(G12V)的氨基酸生物素化变体。使用基于Fmoc的固相肽合成法合成蛋白质,并使用组合的天然化学连接法和异腈介导的激活策略进行组装。我们证明这两个KRas(G12V)对映异构体可以成功折叠和结合核苷酸底物和结合伙伴与可观察到的对映异构。通过证明镜像形式的KRas与其对应的对映体核苷酸三磷酸结合的功能能力,为这项材料的进一步生化研究奠定了基础。特别是,这种蛋白质可以使镜像酵母表面展示实验,以确定清一色d 致癌性KRas的多肽配体,为寻找针对这一具有挑战性的疾病靶标的新疗法提供了有用的工具。
更新日期:2017-05-23
中文翻译:

致癌性KRas(G12V) All- 1和All- d变体的总化学合成和折叠
Ras蛋白是参与细胞增殖和存活调节的必需的GTPases。Ras的致癌突变形式会改变效应子结合和固有的GTPase活性,从而导致下游信号转导的失调。Ras的突变形式涉及大约30%的人类癌症。尽管数十年来一直在努力开发直接的Ras抑制剂,但由于Ras对GTP的亲和力高以及缺乏疏水性结合口袋,长期以来一直被认为是“不可药物的”。在这里,我们报告清一色的全合成升-和清一色d-致癌突变体KRas(G12V)的氨基酸生物素化变体。使用基于Fmoc的固相肽合成法合成蛋白质,并使用组合的天然化学连接法和异腈介导的激活策略进行组装。我们证明这两个KRas(G12V)对映异构体可以成功折叠和结合核苷酸底物和结合伙伴与可观察到的对映异构。通过证明镜像形式的KRas与其对应的对映体核苷酸三磷酸结合的功能能力,为这项材料的进一步生化研究奠定了基础。特别是,这种蛋白质可以使镜像酵母表面展示实验,以确定清一色d 致癌性KRas的多肽配体,为寻找针对这一具有挑战性的疾病靶标的新疗法提供了有用的工具。


























 京公网安备 11010802027423号
京公网安备 11010802027423号