当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium-catalysed atom-economical synthesis of conjugated dienals from terminal acetylenes and acrolein
Chemical Communications ( IF 4.9 ) Pub Date : 2017-05-12 00:00:00 , DOI: 10.1039/c7cc02767b Zoë Hearne 1, 2, 3, 4, 5 , Chao-Jun Li 1, 2, 3, 4, 5
Chemical Communications ( IF 4.9 ) Pub Date : 2017-05-12 00:00:00 , DOI: 10.1039/c7cc02767b Zoë Hearne 1, 2, 3, 4, 5 , Chao-Jun Li 1, 2, 3, 4, 5
Affiliation
|
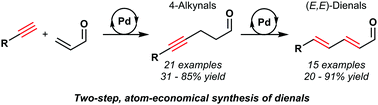
Conjugated (E,E)-dienals are versatile synthetic intermediates owing to their trifunctional, electrophilic nature and the prevalence of the (E,E)-diene in a wide range of functional molecules. It is shown herein that (E,E)-dienals can be readily prepared in two palladium-catalysed steps from simple, unactivated starting materials; terminal acetylenes and acrolein can be coupled via conjugate addition, followed by alkyne isomerisation. This procedure provides a highly atom-economical, redox-neutral and practical method to prepare a range of conjugated (E,E)-dienals in good yields and diastereoselectivities.
中文翻译:

从末端乙炔和丙烯醛中钯催化的原子经济合成共轭二烯
共轭(E,E)二烯化合物由于其三官能,亲电性质以及在各种功能分子中普遍存在(E,E)-二烯而成为通用的合成中间体。在此表明,(E,E)二烯醛可以容易地在两个钯催化的步骤中由简单的未活化的起始原料制备。末端乙炔和丙烯醛可通过共轭加成偶联,然后进行炔烃异构化。该方法提供了一种高度原子经济,氧化还原中性且实用的方法,可以以高收率和非对映选择性制备一系列共轭(E,E)二烯。
更新日期:2017-05-23
中文翻译:

从末端乙炔和丙烯醛中钯催化的原子经济合成共轭二烯
共轭(E,E)二烯化合物由于其三官能,亲电性质以及在各种功能分子中普遍存在(E,E)-二烯而成为通用的合成中间体。在此表明,(E,E)二烯醛可以容易地在两个钯催化的步骤中由简单的未活化的起始原料制备。末端乙炔和丙烯醛可通过共轭加成偶联,然后进行炔烃异构化。该方法提供了一种高度原子经济,氧化还原中性且实用的方法,可以以高收率和非对映选择性制备一系列共轭(E,E)二烯。



























 京公网安备 11010802027423号
京公网安备 11010802027423号