当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
α-Diazo oxime ethers for N-heterocycle synthesis
Chemical Communications ( IF 4.9 ) Pub Date : 2017-05-10 00:00:00 , DOI: 10.1039/c7cc02650a Subin Choi 1, 2, 3, 4 , Sujin Ha 1, 2, 3, 4 , Cheol-Min Park 1, 2, 3, 4
Chemical Communications ( IF 4.9 ) Pub Date : 2017-05-10 00:00:00 , DOI: 10.1039/c7cc02650a Subin Choi 1, 2, 3, 4 , Sujin Ha 1, 2, 3, 4 , Cheol-Min Park 1, 2, 3, 4
Affiliation
|
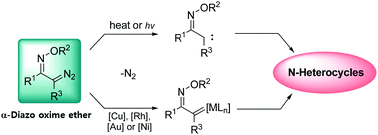
This Feature Article introduces the preparation and synthetic utility of α-diazo oxime ethers. α-Oximino carbenes are useful synthons for N-heterocycles, and can be easily prepared from α-diazo oxime ethers as precursors. We begin with the preparation of α-diazo oxime ethers and their application in [3+2] cycloaddition. It turns out that the nature of metals bound to carbenes plays a crucial role in modulating the reactivity of α-oximino carbenes, in which copper carbenes smoothly react with enamines, whereas the less reactive enol ethers and nitriles require gold carbenes. In Section 3.2, a discussion on N–O and C–H bond activation is presented. Carbenes derived from diazo oxime ethers show unique reactivity towards N–O and C–H bond activation, in which the proximity of the two functionalities, carbene and oxime ether, dictates the preferred reaction pathways toward pyridines, pyrroles, and 2H-azirines. In Section 3.3, the development of tandem reactions based on α-diazo oxime ethers is discussed. The nature of carbenes in which whether free carbenes or metal complexes are involved dissects the pathway and forms different types of 2H-azirines. The 2H-azirine formation turned out to be an excellent platform for the tandem synthesis of N-heterocycles including pyrroles and pyridines. In the last section, we describe the electrophilic activation of 2H-azirines with vinyl carbenes and oximino carbenes. The resulting azirinium species undergo rapid ring expansion rearrangements to form pyridines and pyrazines.
中文翻译:

用于N杂环合成的α-重氮肟醚
该专题文章介绍了α-重氮肟醚的制备和合成用途。α-氧代氨基甲酸酯是N-杂环有用的合成子,并且可以容易地由α-重氮肟醚作为前体制备。我们从α-重氮肟醚的制备及其在[3 + 2]环加成中的应用开始。事实证明,与卡宾结合的金属的性质在调节α-肟基卡宾的反应性中起着至关重要的作用,其中铜卡宾与烯胺平稳地反应,而反应性较低的烯醇醚和腈则需要金卡宾。在第3.2节中,将对N–O和C–H键的激活进行讨论。重氮肟醚衍生的卡宾对N-O和C-H键活化具有独特的反应性,其中两个官能度(卡宾和肟醚)接近,H-叠氮基。在第3.3节中,讨论了基于α-重氮肟肟醚的串联反应的发展。涉及游离的卡宾或金属络合物的卡宾的性质剖析了该途径,并形成了不同类型的2 H-叠氮基。事实证明,2 H-叠氮基形成是串联合成包括吡咯和吡啶的N-杂环的绝佳平台。在最后一节中,我们描述了2 H-叠氮基与乙烯基卡宾和肟基卡宾的亲电活化。所得的叠氮化物类经过快速的环扩展重排以形成吡啶和吡嗪。
更新日期:2017-05-19
中文翻译:

用于N杂环合成的α-重氮肟醚
该专题文章介绍了α-重氮肟醚的制备和合成用途。α-氧代氨基甲酸酯是N-杂环有用的合成子,并且可以容易地由α-重氮肟醚作为前体制备。我们从α-重氮肟醚的制备及其在[3 + 2]环加成中的应用开始。事实证明,与卡宾结合的金属的性质在调节α-肟基卡宾的反应性中起着至关重要的作用,其中铜卡宾与烯胺平稳地反应,而反应性较低的烯醇醚和腈则需要金卡宾。在第3.2节中,将对N–O和C–H键的激活进行讨论。重氮肟醚衍生的卡宾对N-O和C-H键活化具有独特的反应性,其中两个官能度(卡宾和肟醚)接近,H-叠氮基。在第3.3节中,讨论了基于α-重氮肟肟醚的串联反应的发展。涉及游离的卡宾或金属络合物的卡宾的性质剖析了该途径,并形成了不同类型的2 H-叠氮基。事实证明,2 H-叠氮基形成是串联合成包括吡咯和吡啶的N-杂环的绝佳平台。在最后一节中,我们描述了2 H-叠氮基与乙烯基卡宾和肟基卡宾的亲电活化。所得的叠氮化物类经过快速的环扩展重排以形成吡啶和吡嗪。



























 京公网安备 11010802027423号
京公网安备 11010802027423号