当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identification of 4-(Aminomethyl)-6-(trifluoromethyl)-2-(phenoxy)pyridine Derivatives as Potent, Selective, and Orally Efficacious Inhibitors of the Copper-Dependent Amine Oxidase, Lysyl Oxidase-Like 2 (LOXL2)
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-05-04 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00345 Martin W. Rowbottom 1 , Gretchen Bain 1 , Imelda Calderon 1 , Taylor Lasof 1 , David Lonergan 1 , Andiliy Lai 1 , Fei Huang 1 , Janice Darlington 1 , Patricia Prodanovich 1 , Angelina M. Santini 1 , Christopher D. King 1 , Lance Goulet 1 , Kristen E. Shannon 1 , Gina L. Ma 1 , Katherine Nguyen 1 , Deidre A. MacKenna 1 , Jilly F. Evans 1 , John H. Hutchinson 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-05-04 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00345 Martin W. Rowbottom 1 , Gretchen Bain 1 , Imelda Calderon 1 , Taylor Lasof 1 , David Lonergan 1 , Andiliy Lai 1 , Fei Huang 1 , Janice Darlington 1 , Patricia Prodanovich 1 , Angelina M. Santini 1 , Christopher D. King 1 , Lance Goulet 1 , Kristen E. Shannon 1 , Gina L. Ma 1 , Katherine Nguyen 1 , Deidre A. MacKenna 1 , Jilly F. Evans 1 , John H. Hutchinson 1
Affiliation
|
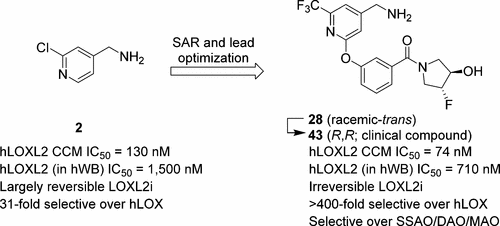
LOXL2 catalyzes the oxidative deamination of ε-amines of lysine and hydroxylysine residues within collagen and elastin, generating reactive aldehydes (allysine). Condensation with other allysines or lysines drives the formation of inter- and intramolecular cross-linkages, a process critical for the remodeling of the ECM. Dysregulation of this process can lead to fibrosis, and LOXL2 is known to be upregulated in fibrotic tissue. Small-molecules that directly inhibit LOXL2 catalytic activity represent a useful option for the treatment of fibrosis. Herein, we describe optimization of an initial hit 2, resulting in identification of racemic-trans-(3-((4-(aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)(3-fluoro-4-hydroxypyrrolidin-1-yl)methanone 28, a potent irreversible inhibitor of LOXL2 that is highly selective over LOX and other amine oxidases. Oral administration of 28 significantly reduced fibrosis in a 14-day mouse lung bleomycin model. The (R,R)-enantiomer 43 (PAT-1251) was selected as the clinical compound which has progressed into healthy volunteer Phase 1 trials, making it the “first-in-class” small-molecule LOXL2 inhibitor to enter clinical development.
中文翻译:

鉴定4-(氨基甲基)-6-(三氟甲基)-2-(苯氧基)吡啶衍生物作为铜依赖性胺氧化酶,赖氨酰氧化酶样2(LOXL2)的有力,选择性和口服有效抑制剂
LOXL2催化胶原蛋白和弹性蛋白中赖氨酸和羟基赖氨酸残基的ε-胺的氧化脱氨反应,生成反应性醛(赖氨酸)。与其他赖氨酸或赖氨酸的缩合驱动分子间和分子内交联的形成,这是ECM重塑的关键过程。该过程的失调可导致纤维化,并且已知LOXL2在纤维化组织中被上调。直接抑制LOXL2催化活性的小分子代表了治疗纤维化的有用选择。在这里,我们描述了初始命中2的优化,导致了外消旋-反式-(3-((4-(氨基甲基)-6-(三氟甲基)吡啶-2-基)氧基)苯基)(3-氟- 4-羟基吡咯烷-1-基)甲酮28,一种有效的LOXL2不可逆抑制剂,对LOX和其他胺氧化酶具有高度选择性。在14天的小鼠肺博来霉素模型中,口服28种可明显减少纤维化。选择(R,R)-对映异构体43(PAT-1251)作为已进入健康的自愿性1期临床试验的临床化合物,使其成为进入临床开发的“一流”小分子LOXL2抑制剂。
更新日期:2017-05-16
中文翻译:

鉴定4-(氨基甲基)-6-(三氟甲基)-2-(苯氧基)吡啶衍生物作为铜依赖性胺氧化酶,赖氨酰氧化酶样2(LOXL2)的有力,选择性和口服有效抑制剂
LOXL2催化胶原蛋白和弹性蛋白中赖氨酸和羟基赖氨酸残基的ε-胺的氧化脱氨反应,生成反应性醛(赖氨酸)。与其他赖氨酸或赖氨酸的缩合驱动分子间和分子内交联的形成,这是ECM重塑的关键过程。该过程的失调可导致纤维化,并且已知LOXL2在纤维化组织中被上调。直接抑制LOXL2催化活性的小分子代表了治疗纤维化的有用选择。在这里,我们描述了初始命中2的优化,导致了外消旋-反式-(3-((4-(氨基甲基)-6-(三氟甲基)吡啶-2-基)氧基)苯基)(3-氟- 4-羟基吡咯烷-1-基)甲酮28,一种有效的LOXL2不可逆抑制剂,对LOX和其他胺氧化酶具有高度选择性。在14天的小鼠肺博来霉素模型中,口服28种可明显减少纤维化。选择(R,R)-对映异构体43(PAT-1251)作为已进入健康的自愿性1期临床试验的临床化合物,使其成为进入临床开发的“一流”小分子LOXL2抑制剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号