当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
7-Substituted 2-Nitro-5,6-dihydroimidazo[2,1-b][1,3]oxazines: Novel Antitubercular Agents Lead to a New Preclinical Candidate for Visceral Leishmaniasis
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-05-01 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00034 Andrew M. Thompson 1 , Patrick D. O’Connor 1 , Andrew J. Marshall 1 , Vanessa Yardley 2 , Louis Maes 3 , Suman Gupta 4 , Delphine Launay 5 , Stephanie Braillard 5 , Eric Chatelain 5 , Scott G. Franzblau 6 , Baojie Wan 6 , Yuehong Wang 6 , Zhenkun Ma 7 , Christopher B. Cooper 7 , William A. Denny 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-05-01 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00034 Andrew M. Thompson 1 , Patrick D. O’Connor 1 , Andrew J. Marshall 1 , Vanessa Yardley 2 , Louis Maes 3 , Suman Gupta 4 , Delphine Launay 5 , Stephanie Braillard 5 , Eric Chatelain 5 , Scott G. Franzblau 6 , Baojie Wan 6 , Yuehong Wang 6 , Zhenkun Ma 7 , Christopher B. Cooper 7 , William A. Denny 1
Affiliation
|
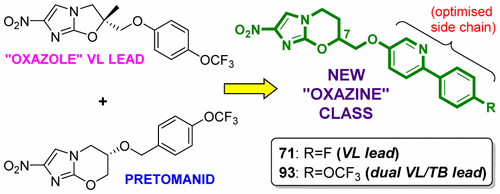
Within a backup program for the clinical investigational agent pretomanid (PA-824), scaffold hopping from delamanid inspired the discovery of a novel class of potent antitubercular agents that unexpectedly possessed notable utility against the kinetoplastid disease visceral leishmaniasis (VL). Following the identification of delamanid analogue DNDI-VL-2098 as a VL preclinical candidate, this structurally related 7-substituted 2-nitro-5,6-dihydroimidazo[2,1-b][1,3]oxazine class was further explored, seeking efficacious backup compounds with improved solubility and safety. Commencing with a biphenyl lead, bioisosteres formed by replacing one phenyl by pyridine or pyrimidine showed improved solubility and potency, whereas more hydrophilic side chains reduced VL activity. In a Leishmania donovani mouse model, two racemic phenylpyridines (71 and 93) were superior, with the former providing >99% inhibition at 12.5 mg/kg (b.i.d., orally) in the Leishmania infantum hamster model. Overall, the 7R enantiomer of 71 (79) displayed more optimal efficacy, pharmacokinetics, and safety, leading to its selection as the preferred development candidate.
中文翻译:

7-取代的2-硝基-5,6-二氢咪唑并[2,1- b ] [1,3]恶嗪:新型抗结核药导致内脏利什曼病的新的临床前候选人
在临床研究药物前mantomanid(PA-824)的备用程序中,从德拉曼胺跳来的脚手架启发了一种新型的有效抗结核药的发现,该药意外地具有明显的抗动塑料样疾病内脏利什曼病(VL)的效用。在确定德拉曼尼德类似物DNDI-VL-2098为VL临床前候选药物后,进一步研究了这种与结构相关的7-取代的2-硝基-5,6-二氢咪唑并[2,1- b ] [1,3]恶嗪类,寻找具有改善的溶解度和安全性的有效备用化合物。从联苯铅开始,通过用吡啶或嘧啶取代一个苯基而形成的生物等排体显示出改善的溶解度和效力,而更多的亲水性侧链降低了VL活性。在利什曼原虫中在小鼠模型中,两种消旋苯基吡啶(71和93)效果更好,前者在利什曼原虫婴儿仓鼠模型中以12.5 mg / kg(出价,口服)提供大于99%的抑制作用。总体而言,71(79)的7 R对映体表现出更佳的功效,药代动力学和安全性,从而使其被选为优选的开发候选药物。
更新日期:2017-05-12
中文翻译:

7-取代的2-硝基-5,6-二氢咪唑并[2,1- b ] [1,3]恶嗪:新型抗结核药导致内脏利什曼病的新的临床前候选人
在临床研究药物前mantomanid(PA-824)的备用程序中,从德拉曼胺跳来的脚手架启发了一种新型的有效抗结核药的发现,该药意外地具有明显的抗动塑料样疾病内脏利什曼病(VL)的效用。在确定德拉曼尼德类似物DNDI-VL-2098为VL临床前候选药物后,进一步研究了这种与结构相关的7-取代的2-硝基-5,6-二氢咪唑并[2,1- b ] [1,3]恶嗪类,寻找具有改善的溶解度和安全性的有效备用化合物。从联苯铅开始,通过用吡啶或嘧啶取代一个苯基而形成的生物等排体显示出改善的溶解度和效力,而更多的亲水性侧链降低了VL活性。在利什曼原虫中在小鼠模型中,两种消旋苯基吡啶(71和93)效果更好,前者在利什曼原虫婴儿仓鼠模型中以12.5 mg / kg(出价,口服)提供大于99%的抑制作用。总体而言,71(79)的7 R对映体表现出更佳的功效,药代动力学和安全性,从而使其被选为优选的开发候选药物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号