当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Targeting of High-Valent Iron-TAML Activators at Hydrocarbons and Beyond
Chemical Reviews ( IF 62.1 ) Pub Date : 2017-05-10 00:00:00 , DOI: 10.1021/acs.chemrev.7b00034 Terrence J. Collins 1 , Alexander D. Ryabov 1
Chemical Reviews ( IF 62.1 ) Pub Date : 2017-05-10 00:00:00 , DOI: 10.1021/acs.chemrev.7b00034 Terrence J. Collins 1 , Alexander D. Ryabov 1
Affiliation
|
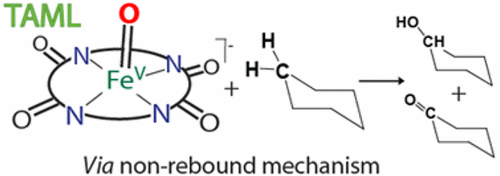
TAML activators of peroxides are iron(III) complexes. The ligation by four deprotonated amide nitrogens in macrocyclic motifs is the signature of TAMLs where the macrocyclic structures vary considerably. TAML activators are exceptional functional replicas of the peroxidases and cytochrome P450 oxidizing enzymes. In water, they catalyze peroxide oxidation of a broad spectrum of compounds, many of which are micropollutants, compounds that produce undesired effects at low concentrations—as with the enzymes, peroxide is typically activated with near-quantitative efficiency. In nonaqueous solvents such as organic nitriles, the prototype TAML activator gave the structurally authenticated reactive iron(V)oxo units (FeVO), wherein the iron atom is two oxidation equivalents above the FeIII resting state. The iron(V) state can be achieved through the intermediacy of iron(IV) species, which are usually μ-oxo-bridged dimers (FeIVFeIV), and this allows for the reactivity of this potent reactive intermediate to be studied in stoichiometric processes. The present review is primarily focused at the mechanistic features of the oxidation by FeVO of hydrocarbons including cyclohexane. The main topic is preceded by a description of mechanisms of oxidation of thioanisoles by FeVO, because the associated studies provide valuable insight into the ability of FeVO to oxidize organic molecules. The review is opened by a summary of the interconversions between FeIII, FeIVFeIV, and FeVO species, since this information is crucial for interpreting the kinetic data. The highest reactivity in both reaction classes described belongs to FeVO. The resting state FeIII is unreactive oxidatively. Intermediate reactivity is typically found for FeIVFeIV; therefore, kinetic features for these species in interchange and oxidation processes are also reviewed. Examples of using TAML activators for C–H bond cleavage applied to fine organic synthesis conclude the review.
中文翻译:

高效铁-TAML活化剂靶向碳氢化合物及其他
过氧化物的TAML活化剂是铁(III)配合物。大环基序中四个去质子化的酰胺氮的连接是TAML的特征,其中大环结构变化很大。TAML激活剂是过氧化物酶和细胞色素P450氧化酶的特殊功能复制品。在水中,它们催化多种化合物的过氧化物氧化,其中许多是微量污染物,这些化合物在低浓度下会产生不良影响,就像酶一样,过氧化物通常以接近定量的效率被活化。在非水溶剂(例如有机腈)中,原型TAML活化剂产生结构上经过验证的反应性铁(V)氧代单元(Fe V O),其中铁原子比Fe高两个氧化当量三,静止状态。铁(V)态可通过铁(IV)物种(通常为μ-氧桥联二聚体(Fe IV Fe IV))的中间体来实现,这使得该强反应性中间体的反应性得以研究。化学计量过程。本综述主要集中在Fe V O氧化包括环己烷在内的碳氢化合物的机理上。的主要课题是通过用Fe苯甲硫醚的氧化机制的描述之前V O,因为相关联的研究提供了有价值的见解Fe的能力V O操作氧化有机分子。本文以Fe III之间的相互转化为总结进行了开头,Fe IV, Fe IV和Fe V O物种,因为此信息对于解释动力学数据至关重要。在上述两个反应类别中,最高的反应性属于Fe VO。静止状态Fe III不具有氧化反应性。通常发现Fe IV Fe IV具有中等反应性;因此,还对这些物质在交换和氧化过程中的动力学特征进行了综述。使用TAML活化剂进行精细有机合成的C–H键裂解的例子总结了本综述。
更新日期:2017-05-10
中文翻译:

高效铁-TAML活化剂靶向碳氢化合物及其他
过氧化物的TAML活化剂是铁(III)配合物。大环基序中四个去质子化的酰胺氮的连接是TAML的特征,其中大环结构变化很大。TAML激活剂是过氧化物酶和细胞色素P450氧化酶的特殊功能复制品。在水中,它们催化多种化合物的过氧化物氧化,其中许多是微量污染物,这些化合物在低浓度下会产生不良影响,就像酶一样,过氧化物通常以接近定量的效率被活化。在非水溶剂(例如有机腈)中,原型TAML活化剂产生结构上经过验证的反应性铁(V)氧代单元(Fe V O),其中铁原子比Fe高两个氧化当量三,静止状态。铁(V)态可通过铁(IV)物种(通常为μ-氧桥联二聚体(Fe IV Fe IV))的中间体来实现,这使得该强反应性中间体的反应性得以研究。化学计量过程。本综述主要集中在Fe V O氧化包括环己烷在内的碳氢化合物的机理上。的主要课题是通过用Fe苯甲硫醚的氧化机制的描述之前V O,因为相关联的研究提供了有价值的见解Fe的能力V O操作氧化有机分子。本文以Fe III之间的相互转化为总结进行了开头,Fe IV, Fe IV和Fe V O物种,因为此信息对于解释动力学数据至关重要。在上述两个反应类别中,最高的反应性属于Fe VO。静止状态Fe III不具有氧化反应性。通常发现Fe IV Fe IV具有中等反应性;因此,还对这些物质在交换和氧化过程中的动力学特征进行了综述。使用TAML活化剂进行精细有机合成的C–H键裂解的例子总结了本综述。


























 京公网安备 11010802027423号
京公网安备 11010802027423号