当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Studies of the Photoinduced Quinone Trimethyl Lock Decaging Process
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-03-24 , DOI: 10.1021/jacs.6b12007 Clinton J. Regan 1 , David P. Walton 1 , Oliver S. Shafaat 1 , Dennis A. Dougherty 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-03-24 , DOI: 10.1021/jacs.6b12007 Clinton J. Regan 1 , David P. Walton 1 , Oliver S. Shafaat 1 , Dennis A. Dougherty 1
Affiliation
|
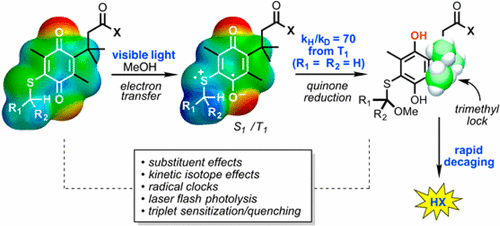
Mechanistic studies of a general reaction that decages a wide range of substrates on exposure to visible light are described. The reaction involves a photochemically initiated reduction of a quinone mediated by an appended thioether. After reduction, a trimethyl lock system incorporated into the quinone leads to thermal decaging. The reaction could be viewed as an electron-transfer initiated reduction of the quinone or as a hydrogen abstraction-Norrish Type II-reaction. Product analysis, kinetic isotope effects, stereochemical labeling, radical clock, and transient absorption studies support the electron transfer mechanism. The differing reactivities of the singlet and triplet states are determined, and the ways in which this process deviates from typical quinone photochemistry are discussed. The mechanism suggests strategies for extending the reaction to longer wavelengths that would be of interest for applications in chemical biology and in a therapeutic setting.
中文翻译:

光致醌三甲基锁老化过程的机理研究
描述了在暴露于可见光时对各种底物进行降解的一般反应的机理研究。该反应涉及由附加的硫醚介导的醌的光化学引发还原。还原后,结合到醌中的三甲基锁定系统导致热分解。该反应可以看作是电子转移引发的醌还原反应,或者是夺氢-Norrish II 型反应。产品分析、动力学同位素效应、立体化学标记、自由基钟和瞬态吸收研究支持电子转移机制。确定了单线态和三线态的不同反应性,并讨论了该过程偏离典型醌光化学的方式。
更新日期:2017-03-24
中文翻译:

光致醌三甲基锁老化过程的机理研究
描述了在暴露于可见光时对各种底物进行降解的一般反应的机理研究。该反应涉及由附加的硫醚介导的醌的光化学引发还原。还原后,结合到醌中的三甲基锁定系统导致热分解。该反应可以看作是电子转移引发的醌还原反应,或者是夺氢-Norrish II 型反应。产品分析、动力学同位素效应、立体化学标记、自由基钟和瞬态吸收研究支持电子转移机制。确定了单线态和三线态的不同反应性,并讨论了该过程偏离典型醌光化学的方式。



























 京公网安备 11010802027423号
京公网安备 11010802027423号