当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Making a Right or Left Choice: Chiral Self-Sorting as a Tool for the Formation of Discrete Complex Structures
Chemical Reviews ( IF 62.1 ) Pub Date : 2017-03-09 00:00:00 , DOI: 10.1021/acs.chemrev.6b00745 Hanna Jędrzejewska 1 , Agnieszka Szumna 1
Chemical Reviews ( IF 62.1 ) Pub Date : 2017-03-09 00:00:00 , DOI: 10.1021/acs.chemrev.6b00745 Hanna Jędrzejewska 1 , Agnieszka Szumna 1
Affiliation
|
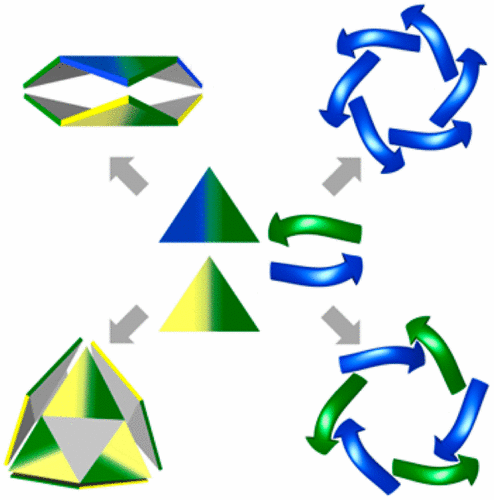
This review discusses chiral self-sorting—the process of choosing an interaction partner with a given chirality from a complex mixture of many possible racemic partners. Chiral self-sorting (also known as chiral self-recognition or chiral self-discrimination) is fundamental for creating functional structures in nature and in the world of chemistry because interactions between molecules of the same or the opposite chirality are characterized by different interaction energies and intrinsically different resulting structures. However, due to the similarity between recognition sites of enantiomers and common conformational lability, high fidelity homochiral or heterochiral self-sorting poses a substantial challenge. Chiral self-sorting occurs among natural and synthetic molecules that leads to the amplification of discrete species. The review covers a variety of complex self-assembled structures ranging from aggregates made of natural and racemic peptides and DNA, through artificial functional receptors, macrocyles, and cages to catalytically active metal complexes and helix mimics. The examples involve a plethora of reversible interactions: electrostatic interactions, π–π stacking, hydrogen bonds, coordination bonds, and dynamic covalent bonds. A generalized view of the examples collected from different fields allows us to suggest suitable geometric models that enable a rationalization of the observed experimental preferences and establishment of the rules that can facilitate further design.
中文翻译:

做出左右选择:手性自分选作为离散复杂结构形成的工具
这篇综述讨论了手性自我分类,即从许多可能的外消旋伙伴的复杂混合物中选择具有给定手性的相互作用伙伴的过程。手性自我分类(也称为手性自我识别或手性自我区分)对于在自然界和化学世界中创建功能结构至关重要,因为具有相同或相反手性的分子之间的相互作用具有不同的相互作用能和本质上不同的结果结构。然而,由于对映异构体的识别位点和常见的构象不稳定性之间的相似性,高保真度的同手性或杂手性自分选提出了巨大的挑战。手性自分类发生在天然和合成分子之间,导致离散物种的扩增。该综述涵盖了各种复杂的自组装结构,从天然和外消旋肽和DNA的聚集体到人工功能受体,大环和笼子,再到具有催化活性的金属络合物和螺旋模拟物。这些示例涉及许多可逆的相互作用:静电相互作用,π-π堆积,氢键,配位键和动态共价键。从不同领域收集的示例的通用视图使我们可以建议合适的几何模型,这些模型可以合理化观察到的实验偏好并建立可以促进进一步设计的规则。和笼子催化活性金属配合物和螺旋模拟物。这些示例涉及许多可逆的相互作用:静电相互作用,π-π堆积,氢键,配位键和动态共价键。从不同领域收集的示例的通用视图使我们可以建议合适的几何模型,这些模型可以合理化观察到的实验偏好并建立可以促进进一步设计的规则。和笼子催化活性金属配合物和螺旋模拟物。这些示例涉及许多可逆的相互作用:静电相互作用,π-π堆积,氢键,配位键和动态共价键。从不同领域收集的示例的通用视图使我们可以建议合适的几何模型,这些模型可以合理化观察到的实验偏好并建立可以促进进一步设计的规则。
更新日期:2017-03-09
中文翻译:

做出左右选择:手性自分选作为离散复杂结构形成的工具
这篇综述讨论了手性自我分类,即从许多可能的外消旋伙伴的复杂混合物中选择具有给定手性的相互作用伙伴的过程。手性自我分类(也称为手性自我识别或手性自我区分)对于在自然界和化学世界中创建功能结构至关重要,因为具有相同或相反手性的分子之间的相互作用具有不同的相互作用能和本质上不同的结果结构。然而,由于对映异构体的识别位点和常见的构象不稳定性之间的相似性,高保真度的同手性或杂手性自分选提出了巨大的挑战。手性自分类发生在天然和合成分子之间,导致离散物种的扩增。该综述涵盖了各种复杂的自组装结构,从天然和外消旋肽和DNA的聚集体到人工功能受体,大环和笼子,再到具有催化活性的金属络合物和螺旋模拟物。这些示例涉及许多可逆的相互作用:静电相互作用,π-π堆积,氢键,配位键和动态共价键。从不同领域收集的示例的通用视图使我们可以建议合适的几何模型,这些模型可以合理化观察到的实验偏好并建立可以促进进一步设计的规则。和笼子催化活性金属配合物和螺旋模拟物。这些示例涉及许多可逆的相互作用:静电相互作用,π-π堆积,氢键,配位键和动态共价键。从不同领域收集的示例的通用视图使我们可以建议合适的几何模型,这些模型可以合理化观察到的实验偏好并建立可以促进进一步设计的规则。和笼子催化活性金属配合物和螺旋模拟物。这些示例涉及许多可逆的相互作用:静电相互作用,π-π堆积,氢键,配位键和动态共价键。从不同领域收集的示例的通用视图使我们可以建议合适的几何模型,这些模型可以合理化观察到的实验偏好并建立可以促进进一步设计的规则。



























 京公网安备 11010802027423号
京公网安备 11010802027423号