当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Flash Vacuum Pyrolysis of Azides, Triazoles, and Tetrazoles
Chemical Reviews ( IF 62.1 ) Pub Date : 2017-02-24 00:00:00 , DOI: 10.1021/acs.chemrev.6b00738 Curt Wentrup 1
Chemical Reviews ( IF 62.1 ) Pub Date : 2017-02-24 00:00:00 , DOI: 10.1021/acs.chemrev.6b00738 Curt Wentrup 1
Affiliation
|
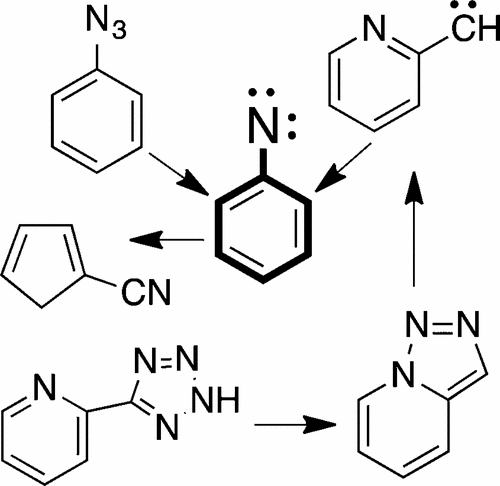
Flash vacuum pyrolysis (FVP) of azides is an extremely valuable method of generating nitrenes and studying their thermal rearrangements. The nitrenes can in many cases be isolated in low-temperature matrices and observed spectroscopically. NH and methyl, alkyl, aralkyl, vinyl, cyano, aryl and N-heteroaryl, acyl, carbamoyl, alkoxycarbonyl, imidoyl, boryl, silyl, phosphonyl, and sulfonyl nitrenes are included. FVP of triazoloazines generates diazomethylazines and azinylcarbenes, which often rearrange to the energetically more stable arylnitrenes. N2 elimination from monocyclic 1,2,3-triazoles can generate iminocarbenes, 1H-azirines, ketenimines, and cyclization products, and 1,2,4-triazoles are precursors of nitrile ylides. Benzotriazoles are preparatively useful precursors of cyanocyclopentadienes, carbazoles, and aza-analogues. FVP of 5-aryltetrazoles can result in double N2 elimination with formation of arylcarbenes or of heteroarylcarbenes, which again rearrange to arylnitrenes. Many 5-substituted and 2,5-disubstituted tetrazoles are excellent precursors of nitrile imines (propargylic, allenic, or carbenic), which are isolable at low temperatures in some cases (e.g., aryl- and silylnitrile imines) or rearrange to carbodiimides. 1,5-Disubstituted tetrazoles are precursors of imidoylnitrenes, which also rearrange to carbodiimides or add intramolecularly to aryl substituents to yield indazoles and related compounds. Where relevant for the mechanistic understanding, pyrolysis under flow conditions or in solution or the solid state will be mentioned. Results of photolysis reactions and computational chemistry complementing the FVP results will also be mentioned in several places.
中文翻译:

叠氮化物,三唑和四唑的快速真空热解
叠氮化物的闪蒸真空热解(FVP)是生成氮烯并研究其热重排的极其有价值的方法。在许多情况下,可以在低温基质中分离出腈,并通过光谱观察。包括NH和甲基,烷基,芳烷基,乙烯基,氰基,芳基和N-杂芳基,酰基,氨基甲酰基,烷氧基羰基,酰亚胺基,硼基,甲硅烷基,膦酰基和磺酰基腈。三唑嗪的FVP生成重氮甲基嗪和叠氮基卡宾,它们通常会重新排列为能量上更稳定的芳基氮化物。单环1,2,3-三唑消除N 2可以生成亚氨基卡宾,1 H-叠氮基,酮亚胺和环化产物以及1,2,4-三唑是腈的前体。苯并三唑是氰基环戊二烯,咔唑和氮杂类似物的制备有用的前体。5-芳基四唑的FVP可导致双N 2消除并形成芳基卡宾或杂芳基卡宾,它们又重新排列成芳基氮烯。许多5取代和2,5-二取代的四唑是腈亚胺(炔丙基,烯丙基或羧甲基)的极好前体,在某些情况下在低温下(例如芳基和甲硅烷基亚胺)可分离或重排成碳二亚胺。1,5-二取代的四唑是亚胺基氮烯的前体,其也重排成碳二亚胺或分子内加成芳基取代基以产生吲唑和相关化合物。在与机械理解有关的地方,将提及在流动条件下或在溶液或固态下的热解。与FVP结果互补的光解反应和计算化学结果也将在数个地方提及。
更新日期:2017-02-24
中文翻译:

叠氮化物,三唑和四唑的快速真空热解
叠氮化物的闪蒸真空热解(FVP)是生成氮烯并研究其热重排的极其有价值的方法。在许多情况下,可以在低温基质中分离出腈,并通过光谱观察。包括NH和甲基,烷基,芳烷基,乙烯基,氰基,芳基和N-杂芳基,酰基,氨基甲酰基,烷氧基羰基,酰亚胺基,硼基,甲硅烷基,膦酰基和磺酰基腈。三唑嗪的FVP生成重氮甲基嗪和叠氮基卡宾,它们通常会重新排列为能量上更稳定的芳基氮化物。单环1,2,3-三唑消除N 2可以生成亚氨基卡宾,1 H-叠氮基,酮亚胺和环化产物以及1,2,4-三唑是腈的前体。苯并三唑是氰基环戊二烯,咔唑和氮杂类似物的制备有用的前体。5-芳基四唑的FVP可导致双N 2消除并形成芳基卡宾或杂芳基卡宾,它们又重新排列成芳基氮烯。许多5取代和2,5-二取代的四唑是腈亚胺(炔丙基,烯丙基或羧甲基)的极好前体,在某些情况下在低温下(例如芳基和甲硅烷基亚胺)可分离或重排成碳二亚胺。1,5-二取代的四唑是亚胺基氮烯的前体,其也重排成碳二亚胺或分子内加成芳基取代基以产生吲唑和相关化合物。在与机械理解有关的地方,将提及在流动条件下或在溶液或固态下的热解。与FVP结果互补的光解反应和计算化学结果也将在数个地方提及。


























 京公网安备 11010802027423号
京公网安备 11010802027423号